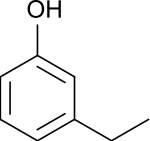
3-Ethylphenol
| |
| Names | |
|---|---|
| Preferred IUPAC name
3-Ethylphenol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.009.663 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H10O | |
| Molar mass | 122.167 g·mol−1 |
| Appearance | colorless liquid |
| Density | 1.0076 g/cm3 |
| Melting point | −4.5 °C (23.9 °F; 268.6 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
3-Ethylphenol is an organic compound with the formula C2H5C6H4OH. It is one of three isomeric ethylphenols. A colorless liquid, it occurs as an impurity in xylenols and as such is used in the production of commercial phenolic resins.[1]
Niche use and occurrence[]
3-Ethylphenol is found in urine samples of female elephants.[2]
It is used as a photographic chemical intermediate and an intermediate for the cyan coupler of photographic paper.[3] It's a tsetse fly attractant. Therefore, it's a kairomone.[4]
References[]
- ^ Fiege, Helmut; Voges, Heinz-Werner; Hamamoto, Toshikazu; Umemura, Sumio; Iwata, Tadao; Miki, Hisaya; Fujita, Yasuhiro; Buysch, Hans-Josef; Garbe (2000). "Phenol Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_313.
- ^ L.E.L. Rasmussen and V. Krishnamurthy (January 2001). "Urinary, temporal gland, and breath odors from Asian elephants of Mudumalai National Park" (PDF). GAJAH, the Journal of the Asian Elephant Specialist Group (20): 1–8.
- ^ Horikawa Y (1998). "Industrialization of the process for cyancoupler intermediate production". Res Dev Rep Sumitomo Chem. 2: 44–48.
- ^ Hitschler, Julia; Grininger, Martin; Boles, Eckhard (2020). "Substrate promiscuity of polyketide synthase enables production of tsetse fly attractants 3-ethylphenol and 3-propylphenol by engineering precursor supply in yeast". Scientific Reports. 10 (1). doi:10.1038/s41598-020-66997-5. ISSN 2045-2322. PMID 32561880.
Categories:
- Alkylphenols
- Aromatic compound stubs