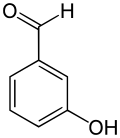
3-Hydroxybenzaldehyde
| |
| Names | |
|---|---|
| Preferred IUPAC name
3-Hydroxybenzaldehyde | |
| Other names
m-Hydroxybenzaldehyde; m-Formylphenol; 3-Formylphenol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.630 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C7H6O2 | |
| Molar mass | 122.123 g·mol−1 |
| Appearance | light-tan crystals |
| Melting point | 100 to 103 °C (212 to 217 °F; 373 to 376 K) |
| Boiling point | 191 °C (376 °F; 464 K) (50 mmHg) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
3-Hydroxybenzaldehyde is one of the three isomers of hydroxybenzaldehyde.
Function[]
3-Hydroxybenzaldehyde exhibits vasculoprotective effects by lowering vascular smooth muscle cell proliferation and endothelial cells inflammation.[1]
Chemistry[]
It has been prepared from 3-nitrobenzaldehyde in a sequence of nitro group reduction, diazotization of the amine, and hydrolysis.[2][3]
Metabolism[]
3-hydroxybenzyl-alcohol dehydrogenase is an enzyme that uses and NADP+ to produce 3-hydroxybenzaldehyde, NADPH and H+.
Uses[]
3-Hydroxybenzaldehyde is used in the synthesis of monastrol.
See also[]
- Salicylaldehyde (2-hydroxybenzaldehyde)
- 4-Hydroxybenzaldehyde
References[]
- ^ Kong, Byung Soo; Im, Soo Jung; Lee, Yang Jong; Cho, Yoon Hee; Do, Yu Ri; Byun, Jung Woo; Ku, Cheol Ryong; Lee, Eun Jig (22 March 2016). "Vasculoprotective Effects of 3-Hydroxybenzaldehyde against VSMCs Proliferation and ECs Inflammation". PLOS ONE. 11 (3): e0149394. Bibcode:2016PLoSO..1149394K. doi:10.1371/journal.pone.0149394. PMC 4803227. PMID 27002821.
- ^ m-HYDROXYBENZALDEHYDE, Organic Syntheses, Coll. Vol. 3, p.453 (1955); Vol. 25, p.55 (1945)
- ^ m-METHOXYBENZALDEHYDE Archived 2012-10-04 at the Wayback Machine, Organic Syntheses, Coll. Vol. 3, p.564 (1955); Vol. 29, p.63 (1949)
Categories:
- Hydroxybenzaldehydes