Acetylide
Acetylide refers to chemical compounds with the chemical formulas MC≡CH and MC≡CM, where M is a metal.[1] The term is used loosely and can refer to substituted acetylides having the general structure RC≡CM (where R is an organic side chain). Acetylides are reagents in organic synthesis. The calcium acetylide commonly called calcium carbide is a major compound of commerce.
Structure and bonding[]
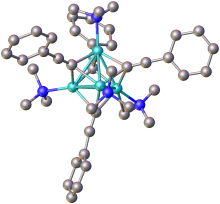
Alkali metal and alkaline earth metal acetylides of the general formula MC≡CM are salt-like Zintl phase compounds, containing C2−
2 ions. Evidence for this ionic character can be seen in the ready hydrolysis of these compounds to form acetylene and metal oxides, there is also some evidence for the solubility of C2−
2 ions in liquid ammonia.[3] The C2−
2 ion has a closed shell ground state of 1Σ+
g, making it isoelectronic to a neutral molecule N2,[4] which may afford it some stability.
Analogous acetylides prepared from other metals, particularly transition metals, show covalent character and are invariably associated with their metal centers. This can be seen in their general stability to water (such as silver acetylide, copper acetylide) and radically different chemical applications.
Acetylides of the general formula RC≡CM (where R = H or alkyl) generally show similar properties to their doubly substituted analogues. In the absence of additional ligands, metal acetylides adopt polymeric structures wherein the acetylide groups are bridging ligands.

Preparation[]
Terminal alkynes are weak acids:[6]
- RC≡CH + R″M ⇌ R″H + RC≡CM
To generate acetylides from acetylene and alkynes relies on the use of organometallic[7] or inorganic[8] superbases in solvents which are less acidic than the terminal alkyne. In early studies liquid ammonia was employed, but ethereal solvents are more common.
Lithium amide,[6] LiHMDS,[9] or organolithium reagents, such as butyllithium,[7] are frequently used to form lithium acetylides:
Sodium or potassium acetylides can be prepared from various inorganic reagents (such as sodium amide)[8] or from their elemental metals, often at room temperature and atmospheric pressure.[6]
Copper(I) acetylide can be prepared by passing acetylene through an aqueous solution of copper(I) chloride because of a low solubility equilibrium.[6] Similarly, silver acetylides can be obtained from silver nitrate.
Calcium carbide is prepared by heating carbon with lime (calcium oxide) at approximately 2,000 °C. A similar process is used to produce lithium carbide.
Reactions[]
Acetylides of the type RC2M are widely used in alkynylations in organic chemistry. They are nucleophiles that add to a variety of electrophilic and unsaturated substrates. A classic application is the Favorskii reaction.
Illustrative is the sequence shown below, ethyl propiolate is deprotonated by n-butyllithium to give the corresponding acetylide. This acetylide adds to the carbonyl center of cyclopentanone. Hydrolytic workup liberate the alkynyl alcohol.[10]

Coupling reactions[]
Acetylides are sometimes intermediates in coupling reactions. Examples include Sonogashira coupling, Cadiot-Chodkiewicz coupling, Glaser coupling and Eglinton coupling.
Hazards[]
Some acetylides are notoriously explosive.[11] Formation of acetylides poses a risk in handling of gaseous acetylene in presence of metals such as mercury, silver or copper, or alloys with their high content (brass, bronze, silver solder).
See also[]
- Ethynyl
- Ethynyl radical
- Diatomic carbon (neutral C2)
- Acetylenediol
References[]
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "acetylides". doi:10.1351/goldbook.A00067
- ^ Schubert, Bernd; Weiss, Erwin (1983). "(PHCCLi)4(tmhda)2, A Polymeric Organolithium Compound with Cubic Li4C4 Structural Units". Angewandte Chemie International Edition in English. 22 (6): 496–497. doi:10.1002/anie.198304961.
- ^ Hamberger, Markus; Liebig, Stefan; Friedrich, Ute; Korber, Nikolaus; Ruschewitz, Uwe (21 December 2012). "Evidence of Solubility of the Acetylide Ion C2−
2: Syntheses and Crystal Structures of K2C2·2 NH3, Rb2C2·2 NH3, and Cs2C2·7 NH3". Angewandte Chemie International Edition. 51 (52): 13006–13010. doi:10.1002/anie.201206349. PMID 23161511. - ^ Sommerfeld, T.; Riss, U.; Meyer, H.-D.; Cederbaum, L. (August 1997). "Metastable C2−
2 Dianion". Physical Review Letters. 79 (7): 1237–1240. Bibcode:1997PhRvL..79.1237S. doi:10.1103/PhysRevLett.79.1237. - ^ Chui, Stephen S. Y.; Ng, Miro F. Y.; Che, Chi-Ming (2005). "Structure Determination of Homoleptic AuI, AgI, and CuI Aryl/Alkylethynyl Coordination Polymers by X-ray Powder Diffraction". Chemistry: A European Journal. 11 (6): 1739–1749. doi:10.1002/chem.200400881. PMID 15669067.
- ^ a b c d Viehe, Heinz Günter (1969). "Chemistry of Acetylenes". Angewandte Chemie (1st ed.). New York: Marcel Dekker. 84 (8): 170–179 & 225–241. doi:10.1002/ange.19720840843.
- ^ a b Midland, M. M.; McLoughlin, J. I.; Werley, Ralph T., Jr. (1990). "Preparation and Use of Lithium Acetylide: 1-Methyl-2-ethynyl-endo-3,3-dimethyl-2-norbornanol". Organic Syntheses. 68: 14. doi:10.15227/orgsyn.068.0014.
- ^ a b Coffman, Donald D. (1940). "Dimethylethhynylcarbinol". Organic Syntheses. 40: 20. doi:10.15227/orgsyn.020.0040.
- ^ Reich, Melanie (August 24, 2001). "Addition of a lithium acetylide to an aldehyde; 1-(2-pentyn-4-ol)-cyclopent-2-en-1-ol". ChemSpider Synthetic Pages (Data Set): 137. doi:10.1039/SP137.
- ^ Midland, M. Mark; Tramontano, Alfonso; Cable, John R. (1980). "Synthesis of alkyl 4-hydroxy-2-alkynoates". The Journal of Organic Chemistry. 45 (1): 28–29. doi:10.1021/jo01289a006.
- ^ Cataldo, Franco; Casari, Carlo S. (2007). "Synthesis, Structure and Thermal Properties of Copper and Silver Polyynides and Acetylides". Journal of Inorganic and Organometallic Polymers and Materials. 17 (4): 641–651. doi:10.1007/s10904-007-9150-3. ISSN 1574-1443. S2CID 96278932.
- Anions
- Functional groups
- Acetylides
![{\displaystyle {\ce {{H-C{\equiv }C-H}+{\overset {butyllithium}{BuLi}}->[{\ce {THF}}][-78^{\circ }{\ce {C}}]{Li-\!{\equiv }\!-H}+BuH}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/7f2a31939e76759509d17aec8c45e1c46780193c)