Alamethicin

| |
| Names | |
|---|---|
| IUPAC name
N-acetyl-2-methylalanyl-L-prolyl-2-methylalanyl-L-alanyl-2-methylalanyl-L-alanyl-L-glutaminyl-2-methylalanyl-L-valyl-2-methylalanylglycyl-L-leucyl-2-methylalanyl-L-prolyl-L-valyl-2-methylalanyl-2-methylalanyl-L-α-glutamyl-N1-[(1S)-1-benzyl-2-hydroxyethyl]-L-glutamamide
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.121.626 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C92H150N22O25 | |
| Molar mass | 1964.31 g/mol |
| Appearance | Off white solid |
| Melting point | 255 to 270 °C (491 to 518 °F; 528 to 543 K) |
| Insoluble | |
| Solubility in DMSO, methanol, ethanol | Soluble |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Alamethicin is a channel-forming peptide antibiotic, produced by the fungus Trichoderma viride. It belongs to peptaibol peptides which contain the non-proteinogenic amino acid residue Aib (2-aminoisobutyric acid). This residue strongly induces formation of alpha-helical structure. The peptide sequence is:
Ac-Aib-Pro-Aib-Ala-Aib-Ala-Gln-Aib-Val-Aib-Gly-Leu-Aib-Pro-Val-Aib-Aib-Glu-Gln-Phl
(Ac = acetyl, Phl = , Aib = 2-Aminoisobutyric acid)
In cell membranes, it forms voltage-dependent ion channels by aggregation of four to six molecules.
Biosynthesis[]
Alamethicin biosynthesis is hypothesized to be catalyzed by alamethicin synthase, a Nonribosomal peptide synthase (NRPS) first isolated in 1975.[2] Although there are several sequences of the alamethicin peptide accepted,[3] evidence suggests these all follow the general NRPS mechanism [4] with small variations at select amino acids.[5] Beginning with the acylation of the N terminal of the first aminoisobutiric acid on the ALM synthase enzyme by Acetyl-CoA,[6] this is followed by the sequential condensation of amino acids by each modular unit of the synthetase.[7] Amino acids are initially adenylated by an “adenylylation” (A) domain before being attached by a thioester bond to an Acyl Carrier Protein-like Peptidyl carrier protein.[8] The growing chain is attached to the amino acid bearing PCP by the "condensation" (C) domain, followed by another round of the same reactions by the next module.[8]
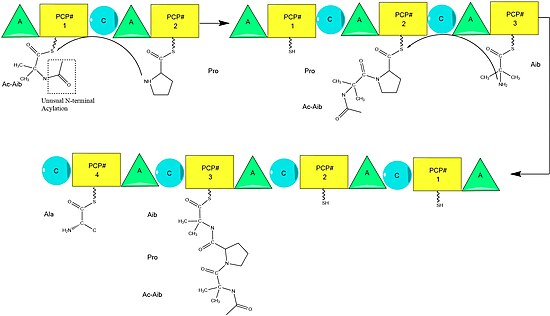
Assembly is completed by the addition of phenylalaninol, an unusual amino acid-like substrate.[9] Following addition of phenylalaninol the completed peptide chain is cleaved by the thioesterase domain, cleaving the thioester bond and leaving an alcohol.

References[]
- ^ Alamethicin product page from Fermentek
- ^ Rindfleisch, H.; Kleinkauf, H. (1976-03-01). "Biosynthesis of alamethicin". FEBS Letters. 62 (3): 276–280. doi:10.1016/0014-5793(76)80074-9. ISSN 0014-5793. PMID 945191.
- ^ Kirschbaum, Jochen; Krause, Corina; Winzheimer, Ruth K.; Brückner, Hans (November–December 2003). "Sequences of alamethicins F30 and F50 reconsidered and reconciled". Journal of Peptide Science. 9 (11–12): 799–809. doi:10.1002/psc.535. ISSN 1075-2617. PMID 14658799. S2CID 25076336.
- ^ Marahiel, Mohamed A.; Stachelhaus, Torsten; Mootz, Henning D. (1997-11-01). "Modular Peptide Synthetases Involved in Nonribosomal Peptide Synthesis". Chemical Reviews. 97 (7): 2651–2674. doi:10.1021/cr960029e. ISSN 0009-2665. PMID 11851476.
- ^ Kleinkauf, H.; Rindfleisch, H. (1975). "Non-ribosomal biosynthesis of the cyclic octadecapeptide alamethicin". Acta Microbiologica Academiae Scientiarum Hungaricae. 22 (4): 411–418. ISSN 0001-6187. PMID 1241650.
- ^ Mohr, H.; Kleinkauf, H. (1978-10-12). "Alamethicin biosynthesis: acetylation of the amino terminus and attachment of phenylalaninol". Biochimica et Biophysica Acta (BBA) - Enzymology. 526 (2): 375–386. doi:10.1016/0005-2744(78)90129-8. ISSN 0006-3002. PMID 568941.
- ^ Weber, Thomas; Marahiel, Mohamed A (January 2001). "Exploring the Domain Structure of Modular Nonribosomal Peptide Synthetases". Structure. 9 (1): –3–R9. doi:10.1016/S0969-2126(00)00560-8. ISSN 0969-2126. PMID 11342140.
- ^ a b Fischbach, Michael A.; Walsh, Christopher T. (August 2006). "Assembly-line enzymology for polyketide and nonribosomal Peptide antibiotics: logic, machinery, and mechanisms". Chemical Reviews. 106 (8): 3468–3496. doi:10.1021/cr0503097. ISSN 0009-2665. PMID 16895337.
- ^ Turner, S. Richard; Voit, Brigitte I.; Mourey, Thomas H. (1993-08-01). "All-aromatic hyperbranched polyesters with C-phenylalaninol and N-acetate end groups: synthesis and characterization". Macromolecules. 26 (17): 4617–4623. Bibcode:1993MaMol..26.4617T. doi:10.1021/ma00069a031. ISSN 0024-9297.
Further reading[]
- Jones, LR; Maddock, SW; Besch, HR Jr (1980). "Unmasking effect of alamethicin on the (Na+,K+)-ATPase, beta-adrenergic receptor-coupled adenylate cyclase, and cAMP-dependent protein kinase activities of cardiac sarcolemmal vesicles". J. Biol. Chem. 255 (20): 9971–9980. doi:10.1016/S0021-9258(18)43488-6. PMID 6253461.
- Explore structures of Alamethicin at the protein data bank
- Alamethicin in Norine
- From "A voltage-gated ion channel model inferred from the crystal structure of alamethicin at 1.5-A resolution." Fox Jr, RO; Richards, FM (1982). "A voltage-gated ion channel model inferred from the crystal structure of alamethicin at 1.5-A resolution". Nature. 300 (5890): 325–30. Bibcode:1982Natur.300..325F. doi:10.1038/300325a0. PMID 6292726. S2CID 4278453.
- Leitgeb, Balázs; Szekeres, András; Manczinger, László; Vágvölgyi, Csaba; Kredics, László (2007-06-01). "The History of Alamethicin: A Review of the Most Extensively Studied Peptaibol". Chemistry & Biodiversity. 4 (6): 1027–1051. doi:10.1002/cbdv.200790095. ISSN 1612-1880. PMID 17589875. S2CID 40886688.
- Polypeptide antibiotics
- Antimicrobial peptides