Amarogentin
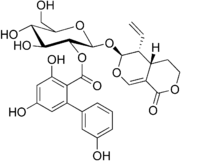 Chemical structure of amarogentin
| |
| Names | |
|---|---|
| Preferred IUPAC name
(2S,3R,4S,5S,6R)-2-{[(4aS,5R,6S)-5-Ethenyl-1-oxo-4,4a,5,6-tetrahydro-1H,3H-pyrano[3,4-c]pyran-6-yl]oxy}-4,5-dihydroxy-6-(hydroxymethyl)oxan-3-yl 3,3′,5-trihydroxy[1,1′-biphenyl]-2-carboxylate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.166.688 |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C29H30O13 | |
| Molar mass | 586.546 g·mol−1 |
| Hazards | |
| GHS labelling:[1] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Amarogentin is a chemical compound found in gentian (Gentiana lutea) or in Swertia chirata.[2]
Gentian root has a long history of use as a herbal bitter in the treatment of digestive disorders and is an ingredient of many proprietary medicines. The bitter principles of gentian root are secoiridoid glycosides amarogentin and . The former is one of the most bitter natural compounds known[3] and is used as a scientific basis for measuring bitterness. In humans, it activates the bitter taste receptor TAS2R50.[4] The biphenylcarboxylic acid moiety is biosynthesized by a polyketide-type pathway, with three units of acetyl-CoA and one unit of 3-hydroxybenzoyl-CoA, this being formed from an early shikimate pathway intermediate and not via cinnamic or benzoic acid.[5]
It also shows an antileishmanial activity in animal models[6] being an inhibitor of topoisomerase I.[7]
References[]
- ^ GHS: sigma-aldrich phl80178 "Not a hazardous substance or mixture according to Regulation (EC) No. 1272/2008"
- ^ Keil*, Michael; Härtle, Birgit; Guillaume, Anna; Psiorz, Manfred (2000). "Production of Amarogentin in Root Cultures of Swertia chirata". Planta Medica. 66 (5): 452–7. doi:10.1055/s-2000-8579. PMID 10909267.
- ^ Heilpflanzen:Gentiana lutea Archived 2009-09-02 at the Wayback Machine (German)
- ^ Behrens, Maik; Brockhoff, Anne; Batram, Claudia; Kuhn, Christina; Appendino, Giovanni; Meyerhof, Wolfgang (2009). "The Human Bitter Taste Receptor hTAS2R50 is Activated by the Two Natural Bitter Terpenoids Andrographolide and Amarogentin". Journal of Agricultural and Food Chemistry. 57 (21): 9860–6. doi:10.1021/jf9014334. PMID 19817411.
- ^ Wang, Chang-Zeng; Maier, Ulrich H.; Eisenreich, Wolfgang; Adam, Petra; Obersteiner, Ingrid; Keil, Michael; Bacher, Adelbert; Zenk, Meinhart H. (2001). "Unexpected Biosynthetic Precursors of Amarogentin − A Retrobiosynthetic13C NMR Study". European Journal of Organic Chemistry. 2001 (8): 1459. doi:10.1002/1099-0690(200104)2001:8<1459::AID-EJOC1459>3.0.CO;2-0.
- ^ Medda, S.; Mukhopadhyay, S; Basu, MK (1999). "Evaluation of the in-vivo activity and toxicity of amarogentin, an antileishmanial agent, in both liposomal and niosomal forms". Journal of Antimicrobial Chemotherapy. 44 (6): 791–4. doi:10.1093/jac/44.6.791. PMID 10590280.
- ^ Ray, Sutapa; Majumder, Hemanta K.; Chakravarty, Ajit K.; Mukhopadhyay, Sibabrata; Gil, Roberto R.; Cordell, Geoffrey A. (1996). "Amarogentin, a Naturally Occurring Secoiridoid Glycoside and a Newly Recognized Inhibitor of Topoisomerase I fromLeishmania donovani". Journal of Natural Products. 59 (1): 27–9. doi:10.1021/np960018g. PMID 8984149.
- Bitter compounds
- Iridoid glycosides
- Topoisomerase inhibitors