Aminoaldehydes and aminoketones
Aminoaldehydes and aminoketones are organic compounds that contain an amine functional group as well as either a aldehyde or ketone functional group. These compounds are important in biology and in chemical synthesis. Because of their bifunctional nature, they have attracted much attention from chemists.
Tertiary amine derivatives[]
Because primary and secondary amines react with aldehydes and ketones, the most common variety of these aminocarbonyl compounds feature tertiary amines. Such compounds are produced by amination of α-haloketones and α-.[1] Examples include cathinones, methadone, molindone, pimeclone, ferruginine, and tropinone.
Secondary amine derivatives[]
Aminoketones containing secondary amines are typically stable when the ketone is located on a ring, e.g. 4-piperidinone, triacetonamine, acridone
Primary amine derivatives[]
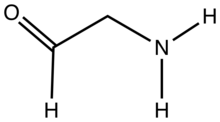
Most members of this class are unstable towards self-condensation, however some important examples do exist as intermediates in biosynthetic pathways e.g. glutamate-1-semialdehyde. The acyclic forms of certain amino sugars also qualify, for instance vancosamine. Aminoacetaldehyde, the simplest member of this subclass, is highly reactive toward self-condensation. Aminoacetaldehyde diethylacetal, (EtO)2CHCH2NH2, is a stable analogue that is commercially available.[2] 2-Aminobenzaldehyde with the formula C6H4(NH2)CHO is a prominent aromatic aminoaldehyde.[3] The compound is unstable with respect to self-condensation

Aminoacetone is a prominent member of this class of compounds. It is unstable under normal laboratory conditions, but the hydrochloride [CH3C(O)CH2NH3]Cl is readily isolable.[5]
Aminoacetone is derived from decarboxylation of alanine. Aminoacetaldehyde is produced by the hydroxylation of taurine.
References[]
- ^ Fisher, Lawrence E.; Muchowski, Joseph M. (1990). "Synthesis of α-Aminoaldehydes and α-Aminoketone. A Review". Organic Preparations and Procedures International. 22 (4): 399–484. doi:10.1080/00304949009356309.
- ^ Amato, Francesco; Marcaccini, Stefano (2005). "2,2-Diethoxy-1-Isocyanoethane". Organic Syntheses. 82: 18. doi:10.15227/orgsyn.082.0018.
- ^ Lee Irvin Smith; J. W. Opie (1948). "o-Aminobenzaldehyde". Org. Synth. 28: 11. doi:10.15227/orgsyn.028.0011.
- ^ Fleischer, E. B.; Klem, E. (1965). "The Structure of a Self-Condensation Product of o-Aminobenzaldehyde in the Presence of Nickel Ions". Inorganic Chemistry. 4 (5): 637–642. doi:10.1021/ic50027a008.
- ^ John D. Hepworth (1965). "Aminoacetone Semicarbazone Hydrochloride". Organic Syntheses. 45: 1. doi:10.15227/orgsyn.045.0001.
- Organic compound stubs
- Amines
- Ketones
- Aldehydes