Avacopan
 | |
| Clinical data | |
|---|---|
| Trade names | Tavneos |
| Other names | CCX168 |
| License data | |
| Routes of administration | By mouth |
| Drug class | Complement C5a receptor antagonist |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
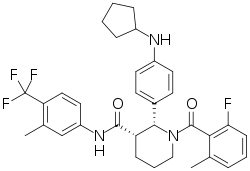
| Formula | C33H35F4N3O2 |
| Molar mass | 581.656 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Avacopan, sold under the brand name Tavneos, is a medication used to treat anti-neutrophil cytoplasmic autoantibody-associated vasculitis.[1][3]
Avacopan was approved for medical use in Japan in September 2021,[2] and in the United States in October 2021.[1][3] It is the first orally-administered inhibitor of the complement C5a receptor approved by the U.S. Food and Drug Administration (FDA).[3]
Society and culture[]
Legal status[]
In November 2021, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Tavneos, intended, in combination with a rituximab or cyclophosphamide regimen, for the treatment of adults with severe, active granulomatosis with polyangiitis or microscopic polyangiitis.[4] The applicant for this medicinal product is Vifor Fresenius Medical Care Renal Pharma France.[4] The EMA considers avacopan to be a first-in-class medicine.[5]
Names[]
Avacopan is the international nonproprietary name (INN).[6]
References[]
- ^ a b c "Tavneos- avacopan capsule". DailyMed. Retrieved 31 October 2021.
- ^ a b "ChemoCentryx Announces Approval in Japan of Tavneos (Avacopan) for the Treatment of ANCA-Associated Vasculitis". ChemoCentryx, Inc. (Press release). 27 September 2021. Retrieved 11 October 2021.
- ^ a b c "ChemoCentryx Announces FDA Approval of Tavneos (avacopan) in ANCA-Associated Vasculitis". ChemoCentryx, Inc. (Press release). 8 October 2021. Retrieved 11 October 2021.
- ^ a b "Tavneos: Pending EC decision". European Medicines Agency. 11 November 2021. Retrieved 12 November 2021. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ "First-in-class medicine recommended for treatment of rare blood vessel inflammation". European Medicines Agency (Press release). 12 November 2021. Retrieved 12 November 2021.
- ^ World Health Organization (2016). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 76". WHO Drug Information. 30 (3). hdl:10665/331020.
Further reading[]
- Jayne DR, Bruchfeld AN, Harper L, Schaier M, Venning MC, Hamilton P, et al. (September 2017). "Randomized Trial of C5a Receptor Inhibitor Avacopan in ANCA-Associated Vasculitis". J Am Soc Nephrol. 28 (9): 2756–2767. doi:10.1681/ASN.2016111179. PMC 5576933. PMID 28400446.
- Jayne DR, Merkel PA, Schall TJ, Bekker P (February 2021). "Avacopan for the Treatment of ANCA-Associated Vasculitis". N Engl J Med. 384 (7): 599–609. doi:10.1056/NEJMoa2023386. PMID 33596356.
- Merkel PA, Jayne DR, Wang C, Hillson J, Bekker P (April 2020). "Evaluation of the Safety and Efficacy of Avacopan, a C5a Receptor Inhibitor, in Patients With Antineutrophil Cytoplasmic Antibody-Associated Vasculitis Treated Concomitantly With Rituximab or Cyclophosphamide/Azathioprine: Protocol for a Randomized, Double-Blind, Active-Controlled, Phase 3 Trial". JMIR Res Protoc. 9 (4): e16664. doi:10.2196/16664. PMC 7175182. PMID 32088663.
- Merkel PA, Niles J, Jimenez R, Spiera RF, Rovin BH, Bomback A, et al. (November 2020). "Adjunctive Treatment With Avacopan, an Oral C5a Receptor Inhibitor, in Patients With Antineutrophil Cytoplasmic Antibody-Associated Vasculitis". ACR Open Rheumatol. 2 (11): 662–671. doi:10.1002/acr2.11185. PMC 7672305. PMID 33128347.
External links[]
- "Avacopan". Drug Information Portal. U.S. National Library of Medicine.
- Clinical trial number NCT02994927 for "A Phase 3 Clinical Trial of CCX168 (Avacopan) in Patients With ANCA-Associated Vasculitis (ADVOCATE)" at ClinicalTrials.gov
- Drugs not assigned an ATC code
- Orphan drugs
- Fluoroarenes
- Trifluoromethyl compounds
- Amides
- Anilines
- Piperidines
- Cyclopentyl compounds
- Antineoplastic and immunomodulating drug stubs