Benzenediazonium chloride
| |
| |
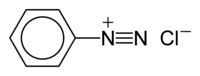
 The benzenediazonium ion
| |
| Names | |
|---|---|
| IUPAC name
Benzenediazonium chloride
| |
| Other names
Phenyldiazonium chloride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.002.584 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H5ClN2 | |
| Molar mass | 140.57 g·mol−1 |
| Appearance | colorless crystals |
| Melting point | decomposes |
| Boiling point | decomposes |
| very good, hydroscopic | |
| Hazards | |
| Main hazards | unstable at temperature exceeding 278K (5C), possibly explosive |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Benzenediazonium chloride is an organic compound with the formula [C6H5N2]Cl. It is a salt of a diazonium cation and chloride. It exists as a colourless solid that is soluble in polar solvents including water. It is the parent member of the aryldiazonium compounds,[1] which are widely used in organic chemistry. Because the salt is unstable, it is not commercially available but is prepared upon demand.
Synthesis[]
This compound is prepared by diazotization of aniline in the presence of hydrochloric acid:[2] The conversion involves in situ production of nitrous acid (HNO2), which reacts with the aniline:
- C6H5NH2 + HNO2 + HCl → [C6H5N2]Cl + 2 H2O
The reactions are conducted at low temperature to minimize decomposition of the diazonium salt. The diazonium salt is not isolated.
Benzenediazonium tetrafluoroborate[]
Benzenediazonium tetrafluoroborate can be obtained from crude benzenediazonium chloride by salt metathesis using tetrafluoroboric acid. The tetrafluoroborate is more stable.[2]
Properties[]
The diazo group (N2) can be replaced by many other groups, usually anions, giving a variety of substituted phenyl derivatives:
- C6H5N2+ + Nu− → C6H5Nu + N2
These transformations are associated with many named reactions including the Schiemann reaction, Sandmeyer reaction, and Gomberg-Bachmann reaction. A wide range of groups that can be used to replace N2 including halide, SH−, CO2H−, OH−. Of considerable practical value in the dye industry are the diazo coupling reactions.
The reaction of phenyldiazonium salts with aniline gives 1,3-diphenyltriazene.[3]
Safety[]
The compound is explosive.[4]
References[]
- ^ March, J. (1992). Advanced Organic Chemistry (4th ed.). New York: J. Wiley and Sons. ISBN 0-471-60180-2.
- ^ a b Flood, D. T. (1933). "Fluorobenzene". Org. Synth. 13: 46. doi:10.15227/orgsyn.013.0046..
- ^ Hartman, W. W.; Dickey, J. B. (1934). "Diazoaminobenzene". Organic Syntheses. 14: 24. doi:10.15227/orgsyn.014.0024.
- ^ Nesmajanow, A. N. (1932). "β-Naphthylmercuric chloride". Organic Syntheses. 12: 54.; Collective Volume, 2, p. 432
- Benzene derivatives
- Diazo compounds
- Chlorides
- Phenyl compounds