Cardiac allograft vasculopathy
| Cardiac allograft vasculopathy | |
|---|---|
 | |
| Coronary arteries | |
| Specialty | Cardiology, angiology |
| Usual onset | After heart transplantation |
| Frequency | Up to 50% (in 10 years) |
Cardiac allograft vasculopathy (CAV) is a common accelerated type of coronary artery disease in people who have had a heart transplantation.[1] This long-term complication affects up to half of heart transplant recipients within 10 years.[2][3] It arises when the blood vessels supplying the transplanted heart gradually narrow and restrict its blood flow, subsequently leading to impairment of the heart muscle or sudden death.[4] The other major causes of death following heart transplantation include graft failure, organ rejection and infection.[5]
People with CAV may present with a wide range of symptoms including tiredness and breathlessness, but there is typically no chest pain. As well as the same risk factors for coronary artery disease due to atherosclerosis, CAV risk factors also include older donors, cytomegalovirus infection and explosive brain death in the donor.[2]
Its pathogenesis involves immunological (innate and adaptive) and nonimmunological factors, with distinct features on histological samples of coronary arteries.[2]
Diagnosis is by regular follow-up and monitoring of the transplanted heart for early signs of disease. This involves invasive diagnostics including coronary angiography and intravascular ultrasound, and non-invasive investigations including dobutamine stress echocardiography, positron emission tomography, computed tomographic angiography (CT angiography) and a variety of biomarkers.[2]
Statins and aspirin are commenced early after transplantation and on detection of CAV. Medications including sirolimus and everolimus can slow disease progression, but a repeat heart transplantation may be needed.[2]
Definition[]
Cardiac allograft vasculopathy is an accelerated type of coronary artery disease in people who have had a heart transplantation.[1]
Signs and symptoms[]
Unlike the ischaemic chest pain in those who have not had a heart transplant, people with CAV typically do not experience chest pain because the donor heart is denervated. A few reinnervate some years later and may develop atypical chest pain.[6] People with CAV may present with a broad spectrum of symptoms including tiredness, nausea, or abdominal discomfort or may have no symptoms at all.[2] Shortness of breath and arrythmias may also occur.[6]
Risk factors[]
Similar to coronary artery disease in those who have not had a heart transplant, risk factors to CAV include high blood pressure, high cholesterol, and diabetes mellitus. Other risk factors exclusive to CAV include older donors, cytomegalovirus infection and circulating antibodies after heart transplantation.[2] The mechanism of donor brain death,[6] particularly explosive brain death in the donor has been shown to be a significant factor. It is probably the combination of injuries to the allograft that determine the risk of developing CAV.[2]
Mechanism[]
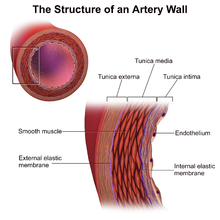
Immunological (innate and adaptive) and nonimmunological factors contribute to the complex pathogenesis of CAV.[2]
In those nontransplanted people who develop coronary artery disease due to atherosclerosis, progression of disease is slow, histological changes are confined mainly to the main coronary arteries and arterial dilatation is observed as a form of compensatory remodelling.[6] However, in CAV, histology specimens typically show concentric thickening of the intimal layer of the main coronary arteries on the surface of the heart and in intramyocardial arteries[2] which can become obliterated within a few years.[6] There is smooth muscle cell migration, foamy macrophages and lymphocytic infiltrates. This can be seen to affect the whole length of the coronary arteries and often the smaller arteries.[2] Calcification does not always occur in CAV and if it does appear, it happens late. The compensatory arterial dilation does not occur in CAV.[6] Unlike in nontransplanted people with coronary artery disease due to atherosclerosis, in CAV occlusion with thrombus of the vessel lumen is rare.[2]
Inflammation and endothelial injury can be triggered by the donor arrest, organ procurement, and allograft ischaemia and reperfusion.[2]
Diagnosis[]
As symptoms are so variable and often absent, diagnosis has been a challenge. Hence, regular follow-up and monitoring of the allograft for early signs of disease is advocated.[2]
Coronary angiography[]
Surveillance is performed by regularly repeating coronary angiography in the cardiac catheterization laboratory, the diagnostic test of choice.[2] This is typically performed annually for the first five years after transplantation.[6] Angiography in CAV characteristically demonstrates diffuse stenoses in large coronary arteries and a reduced number of smaller coronary arteries, also known as "peripheral pruning".[2][3] However, because CAV frequently affects the entire length of the coronary artery, CAV may not be apparent by angiography alone.[2]
Intravascular ultrasound (IVUS)[]
Intravascular ultrasound (IVUS) is more sensitive at reliably detecting subtle changes in the thickness of the intimal layer of the artery walls and provide measurements of artery lumen. Following transplantation, serial measurements are compared to the baseline. A greater than 0.5 mm increase in intimal thickness one year after transplantation is predictive of CAV changes on angiography within five years. The paradoxical reduction in the number of blood vessels, can also be detected by intravascular ultrasound.[2][6]
IVUS, however, tends to be used for research due to its drawbacks of being invasive, requiring the use of contrast material and cost.[6]
Dobutamine stress-echocardiography (DSE)[]
Alternatively, dobutamine stress echocardiography (DSE) is commonly performed and has an 85% sensitivity for the presence of CAV. A negative DSE correlates with a good prognosis.[6]
Other noninvasive diagnostics include positron emission tomography and computed tomographic angiography (CT angiography).[2] In addition, ECGs may show atypical features of ischaemia.[6]
Biomarkers[]
Biomarkers for increased risk of CAV include C-reactive protein, serum brain natriuretic peptide and troponin I have been suggested.[2]
Classification[]
The degree of CAV after heart transplantation has been obtained from a variety of sources including The Cardiac Transplant Research Database, the ISHLT registry and The United Network for Organ Sharing registry.[6]
The International Society for Heart and Lung Transplantation (ISHLT) have formulated and standardized a terminology, based on diagnostic findings, to define the presence and severity of CAV, which in turn reflects prognosis.[6][8] The severity of CAV is defined by the degree of narrowing of the coronary arteries and the presence of restrictive heart disease.[6]
| Code | Severity | Diagnostic findings |
|---|---|---|
| ISHLT CAV0 | Not significant | No detectable lesions on angiography |
| ISHLT CAV1 | Mild | Angiographic left main (LM) |