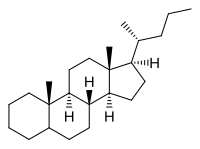
Cholane
| |
| Names | |
|---|---|
| IUPAC name
Cholane[1]
| |
| Preferred IUPAC name
(1R,3aS,3bR,5aΞ,9aS,9bS,11aR)-9a,11a-Dimethyl-1-[(2R)-pentan-2-yl]hexadecahydro-1H-cyclopenta[a]phenanthrene | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C24H42 | |
| Molar mass | 330.59 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Cholane is a triterpene which can exist as either of two stereoisomers, 5α-cholane and 5β-cholane. Its name is derived from the Greek word for bile (χολή, chole) in reference to its original discovery from the bile of the American bullfrog (Rana catesbeiana).[2] The compound itself has no known uses. However, various functionalized analogues are produced by plants and animals, typically in the form of sterols, steroids and bile acids (e.g. cholic acid).
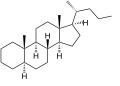
5α-Cholane
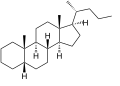
5β-Cholane
See also[]
References[]
- ^ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 1528. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ^ Kurauti, Yukiti; Kazuno, Taro (January 1939). "Tetraoxycholan, Trioxycholen und Trioxy-bis-norsterocholansäure aus der Galle von Rana Catesbina Shaw". Hoppe-Seyler's Zeitschrift für physiologische Chemie (in German). 262 (1–2): 53–60. doi:10.1515/bchm2.1939.262.1-2.53.
External links[]
- Cholanes at the US National Library of Medicine Medical Subject Headings (MeSH)
Categories:
- Triterpenes
- Cholanes
- Cyclopentanes

