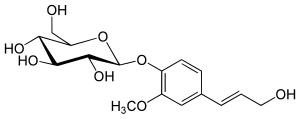
Coniferin
| |
| Names | |
|---|---|
| Preferred IUPAC name
(2R,3S,4S,5R,6S)-2-(Hydroxymethyl)-6-{4-[(1E)-3-hydroxyprop-1-en-1-yl]-2-methoxyphenoxy}oxane-3,4,5-triol | |
| Other names
• β-D-Glucopyranoside 4-(3-hydroxy-1-propenyl)-2-methoxyphenyl
• Coniferyl alcohol β-D-glucoside | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.230.647 |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C16H22O8 | |
| Molar mass | 342.35 g/mol |
| Melting point | 186 °C (367 °F; 459 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Coniferin is a glucoside of coniferyl alcohol. This white crystalline solid is a metabolite in conifers, serving as an intermediate in cell wall lignification, as well as having other biological roles. It can also be found in the water root extract of Angelica archangelica subsp. litoralis.[1]
Vanillin was first synthesized from coniferin by chemists Ferdinand Tiemann and Wilhelm Haarmann.
References[]
This article needs additional citations for verification. (September 2013) |
- ^ Dihydrofurocoumarin glucosides from Angelica archangelica and Angelica silvestris. John Lemmich, Svend Havelund and Ole Thastrup, Phytochemistry, 1983, Volume 22, Issue 2, Pages 553–555, doi:10.1016/0031-9422(83)83044-1
Categories:
- Monolignol glucosides
- Aromatic compound stubs