Cuprate
Cuprate loosely refers to a material that can be viewed as containing anionic copper complexes. Examples include tetrachloridocuprate ([CuCl4]2−), the superconductor YBa2Cu3O7, and the organocuprates (e.g., dimethylcuprate [Cu(CH3)2]−).[1] The term cuprates derives from the Latin word for copper, cuprum. The term is mainly used in three contexts: oxide materials, anionic coordination complexes, and anionic organocopper compounds.
Oxides[]
One of the simplest oxide-based cuprates is the copper(III) oxide KCuO2. This species can be viewed as the K+ salt of the polyanion [CuO−
2]n. As such the material is classified as a cuprate. This dark blue diamagnetic solid is produced by heating potassium peroxide and copper(II) oxide in an atmosphere of oxygen:[2]
- K2O2 + 2 CuO → 2 KCuO2
Coordination complexes[]
Copper forms many anionic coordination complexes with negatively charged ligands such as cyanide, hydroxide, halides, as well as alkyls and aryls.
Copper(I)[]
Cuprates containing copper(I) tend to be colorless, reflecting their d10 configuration. Structures range from linear 2-coordinate, trigonal planar, and tetrahedral. Examples include dichloro and trichlorocuprates, i.e., linear [CuCl2]− and trigonal planar [CuCl3]2−.[3] Cyanide gives analogous complexes but also the trianionic tetracyanocuprate(I), [Cu(CN)4]3−.[4] Dicyanocuprate(I) exists in both molecular or polymeric motifs, depending on the countercation.[5]
Copper(II)[]

The chlorocuprates include trichlorocuprate(II) [CuCl3]−, which is dimeric, square-planar tetrachlorocuprate(II) [CuCl4]2−, and pentachlorocuprate(II) [CuCl5]3−.[1][6] 3-Coordinate chlorocuprate(II) complexes are rare.[7]
Tetrachlorocuprate(II) complexes tend to adopt flattened tetrahedral geometry with orange colors.[8][9][10][11]
Sodium tetrahydroxycuprate (Na2[Cu(OH)4]) is an example of a homoleptic (all ligands being the same) hydroxide complex.[12]
- Cu(OH)2 + 2 NaOH → Na2Cu(OH)4
Copper(III) and copper(IV)[]
Hexafluorocuprate(III) [CuF6]3− and hexafluorocuprate(IV) [CuF6]2− are rare examples of copper(III) and copper(IV) complexes. They are strong oxidizing agents.
Organic cuprates[]
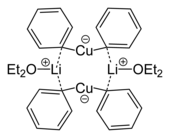
Cuprates have a role in organic synthesis. They are invariably Cu(I), although Cu(II) or even Cu(III) intermediates are invoked in some mechanisms. Organic cuprates often have the idealized formulas [CuR2]− and [CuR3]2−, where R is an alkyl or aryl. These reagents find use as nucleophilic alkylating reagents.[14]
See also[]
References[]
- ^ a b Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ G. Brauer, ed. (1963). "Potassium Cuprate (III)". Handbook of Preparative Inorganic Chemistry. 1 (2nd ed.). NY: Academic Press. p. 1015.
- ^ Stricker, Marion; Linder, Thomas; Oelkers, Benjamin; Sundermeyer, Jörg (2010). "Cu(I)/(II) based catalytic ionic liquids, their metallo-laminate solid state structures and catalytic activities in oxidative methanol carbonylation". Green Chemistry. 12 (9): 1589. doi:10.1039/c003948a.
- ^ Kroeker, Scott; Wasylishen, Roderick E. (1999). "Article". Canadian Journal of Chemistry. 77 (11): 1962–1972. doi:10.1139/v99-181.
- ^ Bowmaker, Graham A.; Hartl, Hans; Urban, Victoria (2000). "Crystal Structures and Vibrational Spectroscopy of [NBu4][Cu(CN)X] (X = Br, I) and [NBu4][Cu3(CN)4]·CH3CN". Inorganic Chemistry. 39 (20): 4548–4554. doi:10.1021/ic000399s.
- ^ Willett, Roger D.; Butcher, Robert E.; Landee, Christopher P.; Twamley, Brendan (2006). "Two Halide Exchange in Copper(II) Halide Dimers: (4,4′-Bipyridinium)Cu2Cl6−x BRX". Polyhedron. 25 (10): 2093–2100. doi:10.1016/j.poly.2006.01.005.
- ^ Hasselgren, Catrin; Jagner, Susan; Dance, Ian (2002). "Three-Coordinate [CuIIX3]− (X = Cl, Br), Trapped in a Molecular Crystal". Chemistry – A European Journal. 8 (6): 1269–1278. doi:10.1002/1521-3765(20020315)8:6<1269::AID-CHEM1269>3.0.CO;2-9.
- ^ Mahoui, A.; Lapasset, J.; Moret, J.; Saint Grégoire, P. (1996). "Tetraethylammonium Tetramethylammonium Tetrachlorocuprate(II), [(C2H5)4N][(CH3)4N][CuCl4]". Acta Crystallographica Section C. 52 (11): 2674–2676. doi:10.1107/S0108270196009031.
- ^ Guillermo Mínguez Espallargas, Lee Brammer, Jacco van de Streek, Kenneth Shankland, Alastair J. Florence, and Harry Adams (2006). "Reversible Extrusion and Uptake of HCl Molecules by Crystalline Solids Involving Coordination Bond Cleavage and Formation". J. Am. Chem. Soc. 128 (30): 9584–9585. doi:10.1021/ja0625733. PMID 16866484.CS1 maint: uses authors parameter (link)
- ^ Kelley, A.; Nalla, S.; Bond, M. R. (2015). "The square-planar to flattened-tetrahedral CuX42− (X = Cl, Br) structural phase transition in 1,2,6-trimethylpyridinium salts". Acta Crystallogr. B. 71 (Pt 1): 48–60. doi:10.1107/S205252061402664X. PMID 25643715.
- ^ Egon Wiberg; Nils Wiberg; Arnold Frederick Holleman (2001). Inorganic Chemistry. Academic Press. pp. 1252–1264. ISBN 0-12-352651-5.
- ^ Brauer, G., ed. (1963). "Sodium Tetrahydroxocuprate(II)". Handbook of Preparative Inorganic Chemistry. 1 (2nd ed.). New York, NY: Academic Press. p. 1015.
- ^ Lorenzen, Nis Peter; Weiss, Erwin (1990). "Synthesis and Structure of a Dimeric Lithium Diphenylcuprate:[{Li(OEt2)}(CuPh2)]2". Angewandte Chemie International Edition in English. 29 (3): 300. doi:10.1002/anie.199003001.
- ^ Louis S. Hegedus (1999). Transition metals in the synthesis of complex organic molecules. University Science Books. pp. 61–65. ISBN 1-891389-04-1.
- Copper compounds
- Anions