Curli
| Curli secretion channel | |||||||
|---|---|---|---|---|---|---|---|
 | |||||||
| Identifiers | |||||||
| Organism | |||||||
| Symbol | CsgG | ||||||
| Entrez | 945619 | ||||||
| PDB | 3X2R | ||||||
| RefSeq (mRNA) | NC_000913.3 | ||||||
| RefSeq (Prot) | NP_415555.1 | ||||||
| UniProt | P0AEA2 | ||||||
| Other data | |||||||
| Chromosome | Genomic: 1.1 - 1.1 Mb | ||||||
| |||||||
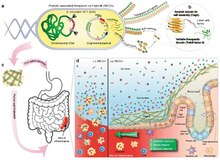
The Curli protein is a type of amyloid fiber produced by certain strains of enterobacteria. They are extracellular fibers located on bacteria such as E. coli and Salmonella.[2] These fibers serve to promote cell community behavior through biofilm formation in the extracellular matrix. Amyloid is associated with human neurodegenerative diseases such as Alzheimer's disease.[3] The study of curli may help to understand human diseases thought to arise from improper amyloid fiber formation.[2] The curli pili are generally assembled through the .[4]
Gene regulation[]
Curli fibers, and thus the curli protein, are coded by two operons, the csgBAC and csgDEFG operon, in total containing seven genes.[5][6] The csgBAC operon is responsible for the coding the three proteins CsgB, CsgA, and CsgC, all responsible for either the major subunit formation within the curli fiberal, or the inhibition of it.[6] The csgDEFG operon codes for proteins CsgD, CsgE, CsgF, and CsgG, responsible for the assembly, translocation, and regulation of the curli protein.[6]
CsgD is a positive transcriptional regulator of the csgBA operon. The CsgD protein is the transcription regulator and positively regulates the csgBA operon but does not regulate its own expression. The csgBA operon encodes the major structural subunit of curli, CsgA, as well as the nucleator protein CsgB. Further research still needs to be conducted in order to see what stimulates CsgD expression or activity but some evidence supports that the activation of the protein is caused by the phosphorylation of an aspartic acid residue of the N-terminal receiver domain. Since CsgD must be present for csgBA promoter activity, it is therefore shown that regulators of CsgD expression also influence the expression of csgBA.[2]
Many environmental cues play a role in Curli gene expression. Growth at a temperature below 30 degrees celsius promotes Curli gene expression.[2] Additionally, when there is a lack of salt and nutrients such as nitrogen, phosphate and iron, Curli gene expression is stimulated.[2]
Function[]
Amyloids have been linked to illnesses such as Alzheimer's, Parkinson's, Huntington's, lupus, among many more.[7][8] Additionally, the Curli protein is considered a PAMP (Pathogen-associated molecular pattern) and so Curli's beta sheet structure acts on the innate immune system, activating the TLR2 (Toll-Like Receptor 2).[7] This then causes a downstream response by a producing proinflammatory response where proinflammatory cytokines and chemokines are recruited to initiation and inflammation response.[7]
Curli, however, has other functions (thus being coined a "functional amyloid"[8]) including being a major component in the biofilm generated by gram-negative bacterial such as E. coli and Salmonella.[9][7][8] These biofilms allow gram-negative bacteria to better colonize in a given environment, protecting them from oxidative stress and dehydration.[9][7] These biofilms, however, call for much concern. As these biofilms allow for the bacteria to survive chemical and physical stressors within their environment, not only does it make patients more susceptible to infection when using shared appliances, but Curli and other biofilms have shown to reduce the infected individuals immune response and antimicrobials.[9] Curli proteins and biofilms alike are very resistant to chemical stressors to a point where stronger pretreatment is required in order for Curli to degrade or dissolve in sodium dodecyl sulphate (SDS).[9]
Structure[]
The curli protein's main components (subunits) consist of the CsgA and CsgB protein.
CsgA[]
CsgA is the major subunit of the Curli protein at 13.1 kiloDalton. This protein consists of three domains which have a tendency to aggregate to form amyloid fibrils, a single peptide, a 22 amino acid N terminal sequence (used for secretion) and an amyloid core domain at the C-terminal sequence.[9][8] Furthermore, the amyloid core domain is composed of 5 repeating (yet not exact) sequences revolving around the sequence: Ser-X5-Gln-X-Gly-X-Gly-Asn-X-Ala-X3-Gln.[8] This repeating sequence is the characteristic subunit that forms the aggregatable β-sheet.[8]
CsgB[]
CsgB, also known as the minor subunit, is required for the nucleation and organization of CsgA into a Curli fiber on the cells surface.[7] CsgB has a very similar repeating sequence to that of CsgA with the expectation that one of the 5 repeating sequences does contains additional amino acids, known as Lys133, Arg140, Arg14, and Arg151.[9] This change in the final subunit (known as the R5 subunit) is required. Without the presence of the R5 subunit, or the changes within the subunit, CsgA can not properly form on the cell surface.[9][8]
CsgC[]
The CsgC subunit only recently was discovered to prevent the aggregation and polymerization of the CsgA protein. Without it, there is a chance for amyloid fibril formation and eventual cell death.[7] Multiple experiments isolating CsgC away from the CsgA and CsgB subunit caused for CsgA to aggregate into fibrils, and therefore possibly leading to the downstream effects on illnesses such as Alzheimer's.[9] The molar ratio required for CsgC to inhibit CsgA is 1:500, thus meaning only 1 CsgC protein is required to inhibit 500 CsgA proteins from forming amyloid fibril structures.[9][10] It is hypothesized that CsgC is therefore considered a chaperone as it prevents further CsgA nucleation, rather allowing CsgA to form into its proper structure instead of aggregating.[9]
References[]
- ^ Praveschotinunt P, Duraj-Thatte AM, Gelfat I, Bahl F, Chou DB, Joshi NS (December 2019). "Engineered E. coli Nissle 1917 for the delivery of matrix-tethered therapeutic domains to the gut". Nature Communications. 10 (1): 5580. doi:10.1038/s41467-019-13336-6. PMC 6898321. PMID 31811125.
- ^ a b c d e Barnhart MM, Chapman MR (2006). "Curli biogenesis and function". Annual Review of Microbiology. 60: 131–47. doi:10.1146/annurev.micro.60.080805.142106. PMC 2838481. PMID 16704339.
- ^ Chapman MR, Robinson LS, Pinkner JS, Roth R, Heuser J, Hammar M, et al. (February 2002). "Role of Escherichia coli curli operons in directing amyloid fiber formation". Science. 295 (5556): 851–5. Bibcode:2002Sci...295..851C. doi:10.1126/science.1067484. PMC 2838482. PMID 11823641.
- ^ Hammar M, Bian Z, Normark S (June 1996). "Nucleator-dependent intercellular assembly of adhesive curli organelles in Escherichia coli". Proceedings of the National Academy of Sciences of the United States of America. 93 (13): 6562–6. doi:10.1073/pnas.93.13.6562. PMC 39064. PMID 8692856.
- ^ Van Gerven N, Klein RD, Hultgren SJ, Remaut H (November 2015). "Bacterial amyloid formation: structural insights into curli biogensis". Trends in Microbiology. 23 (11): 693–706. doi:10.1016/j.tim.2015.07.010. PMC 4636965. PMID 26439293.
- ^ a b c Tursi SA, Tükel Ç (December 2018). "Curli-Containing Enteric Biofilms Inside and Out: Matrix Composition, Immune Recognition, and Disease Implications". Microbiology and Molecular Biology Reviews. 82 (4): e00028–18, /mmbr/82/4/e00028–18.atom. doi:10.1128/MMBR.00028-18. PMC 6298610. PMID 30305312.
- ^ a b c d e f g Tursi SA, Tükel Ç (December 2018). "Curli-Containing Enteric Biofilms Inside and Out: Matrix Composition, Immune Recognition, and Disease Implications". Microbiology and Molecular Biology Reviews. 82 (4): e00028–18, /mmbr/82/4/e00028–18.atom. doi:10.1128/MMBR.00028-18. PMC 6298610. PMID 30305312.
- ^ a b c d e f g Evans ML, Chapman MR (August 2014). "Curli biogenesis: order out of disorder". Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 1843 (8): 1551–8. doi:10.1016/j.bbamcr.2013.09.010. PMC 4243835. PMID 24080089.
- ^ a b c d e f g h i j Van Gerven N, Klein RD, Hultgren SJ, Remaut H (November 2015). "Bacterial amyloid formation: structural insights into curli biogensis". Trends in Microbiology. 23 (11): 693–706. doi:10.1016/j.tim.2015.07.010. PMC 4636965. PMID 26439293.
- ^ Evans ML, Chorell E, Taylor JD, Åden J, Götheson A, Li F, et al. (February 2015). "The bacterial curli system possesses a potent and selective inhibitor of amyloid formation". Molecular Cell. 57 (3): 445–55. doi:10.1016/j.molcel.2014.12.025. PMC 4320674. PMID 25620560.
Further reading[]
- Dema B, Charles N (January 2016). "Autoantibodies in SLE: Specificities, Isotypes and Receptors". Antibodies. 5 (1): 2. doi:10.3390/antib5010002. PMC 6698872. PMID 31557984.
- Gallo PM, Rapsinski GJ, Wilson RP, Oppong GO, Sriram U, Goulian M, et al. (June 2015). "Amyloid-DNA Composites of Bacterial Biofilms Stimulate Autoimmunity". Immunity. 42 (6): 1171–84. doi:10.1016/j.immuni.2015.06.002. PMC 4500125. PMID 26084027.
- Kono DH, Baccala R, Theofilopoulos AN (December 2013). "TLRs and interferons: a central paradigm in autoimmunity". Current Opinion in Immunology. 25 (6): 720–7. doi:10.1016/j.coi.2013.10.006. PMC 4309276. PMID 24246388.
- Tursi SA, Lee EY, Medeiros NJ, Lee MH, Nicastro LK, Buttaro B, et al. (April 2017). "Bacterial amyloid curli acts as a carrier for DNA to elicit an autoimmune response via TLR2 and TLR9". PLOS Pathogens. 13 (4): e1006315. doi:10.1371/journal.ppat.1006315. PMC 5406031. PMID 28410407.
- Bacterial proteins
- Biochemistry stubs