Cyanobacterial morphology

A–C: Microcoleus steenstrupii D–E: Tolypothrix desertorum F: Scytonema cf. calcicola G: S. cf. calcicola H: S. cf. c alcicola
Scale bar =10 µm
Cyanobacteria are a large and diverse phylum of bacteria defined by their unique combination of pigments and their ability to perform oxygenic photosynthesis.[1][2]
Cyanobacteria often live in colonial aggregates that can take a multitude of forms.[2] Of particular interest among the many species of cyanobacteria are those that live colonially in long trichomes of hundreds to thousands of cells.[2] These are the filamentous species, which often dominate the upper layers of microbial mats found in extreme environments such as hot springs, hypersaline water, deserts and polar regions,[3] as well as being widely distributed in more mundane environments.[2]
Many filamentous species are also motile, gliding along their long axis, and displaying photomovement by which a trichome modulates its gliding according to the incident light. The latter has been found to play an important role in guiding the trichomes to optimal lighting conditions, which can either inhibit the cells if the incident light is too weak, or damage the cells if too strong.[2]
Overview[]
Cyanobacteria present remarkable variability in terms of morphology: from unicellular and colonial to filamentous forms. Filamentous forms exhibit functional cell differentiation such as heterocysts (for nitrogen fixation), akinetes (resting stage cells), and hormogonia (reproductive, motile filaments). These, together with the intercellular connections they possess, are considered the first signs of multicellularity.[4][5][6][7]
Many cyanobacteria form motile filaments of cells, called hormogonia, that travel away from the main biomass to bud and form new colonies elsewhere.[8][9] The cells in a hormogonium are often thinner than in the vegetative state, and the cells on either end of the motile chain may be tapered. To break away from the parent colony, a hormogonium often must tear apart a weaker cell in a filament, called a necridium.
Single cell species[]
Each individual cell (each single cyanobacterium) typically has a thick, gelatinous cell wall.[10] They lack flagella, but hormogonia of some species can move about by gliding along surfaces.[11]
Colonial species[]
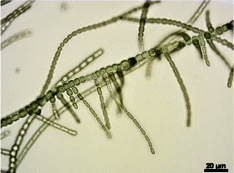
True branching phenotype of a Fischerella thermalis colony

Lyngbya species form long, unbranching filaments inside rigid mucilaginous sheaths which can form tangles or mats, intermixed with other phytoplankton species

Cyanobacterial colony of Lyngbya majuscula
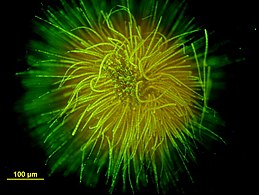
Ball-shaped colony of Gloeotrichia echinulata stained with SYTOX

Colonies of Nostoc pruniforme

Colonial cyanobacteria Stratonostoc
Filamentous species[]
Some filamentous species can differentiate into several different cell types:
- vegetative cells – the normal, photosynthetic cells that are formed under favorable growing conditions
- akinetes – climate-resistant spores that may form when environmental conditions become harsh
- thick-walled heterocysts – which contain the enzyme nitrogenase vital for nitrogen fixation[12][13][14] in an anaerobic environment due to its sensitivity to oxygen.[14]
Many of the multicellular filamentous forms of Oscillatoria are capable of a waving motion; the filament oscillates back and forth. In water columns, some cyanobacteria float by forming gas vesicles, as in archaea.[15] These vesicles are not organelles as such. They are not bounded by lipid membranes but by a protein sheath.
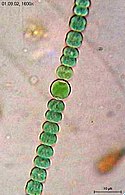
Anabaena sperica

Anabaena is used as a model organism to study simple vision[16]

Helical filaments of cyanobacteria
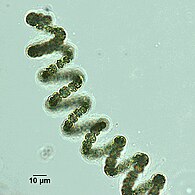
Helical filament from Dolichospermum
Nassula consuming oscillatoria through its cytopharyngeal basket
Diversity[]

scale bars about 10 µm



Heterocysts[]
Heterocysts are specialized nitrogen-fixing cells formed during nitrogen starvation by some filamentous cyanobacteria, such as Nostoc punctiforme, Cylindrospermum stagnale, and Anabaena sphaerica.[17] They fix nitrogen from atmospheric N2 using the enzyme nitrogenase, in order to provide the cells in the filament with nitrogen for biosynthesis.[18]
Movement[]

Cyanobacteria have strict light requirements. Too little light can result in insufficient energy production, and in some species may cause the cells to resort to heterotrophic respiration.[3] Too much light can inhibit the cells, decrease photosynthesis efficiency and cause damage by bleaching. UV radiation is especially deadly for cyanobacteria, with normal solar levels being significantly detrimental for these microorganisms in some cases.[19][20][2]
Filamentous cyanobacteria that live in microbial mats often migrate vertically and horizontally within the mat in order to find an optimal niche that balances their light requirements for photosynthesis against their sensitivity to photodamage. For example, the filamentous cyanobacteria Oscillatoria sp. and Spirulina subsalsa found in the hypersaline benthic mats of Guerrero Negro, Mexico migrate downwards into the lower layers during the day in order to escape the intense sunlight and then rise to the surface at dusk.[21] In contrast, the population of Microcoleus chthonoplastes found in hypersaline mats at Salins-de-Giraud, Camargue, France migrate to the upper layer of the mat during the day and are spread homogenously through the mat at night.[22] An in vitro experiment using P. uncinatum also demonstrated this species' tendency to migrate in order to avoid damaging radiation.[19][20] These migrations are usually the result of some sort of photomovement, although other forms of taxis can also play a role.[23][2]
Many species of cyanobacteria are capable of gliding. Gliding is a form of cell movement that differs from crawling or swimming in that it does not rely on any obvious external organ or change in cell shape and it occurs only in the presence of a substrate.[24][25] Gliding in filamentous cyanobacteria appears to be powered by a "slime jet" mechanism, in which the cells extrude a gel that expands quickly as it hydrates providing a propulsion force,[26][27] although some unicellular cyanobacteria use type IV pili for gliding.[28] Individual cells in a trichome have two sets of pores for extruding slime. Each set is organized in a ring at the cell septae and extrudes slime at an acute angle.[29] The sets extrude slime in opposite directions and so only one set is likely to be activated during gliding. An alternative hypothesis is that the cells use contractive elements that produce undulations running over the surface inside the slime tube like an earthworm.[30] The trichomes rotate in a spiral fashion, the angle of which corresponds with the pitch angle of Castenholz's contractile trichomes.[2]
The cells appear to coordinate their gliding direction by an electrical potential that establishes polarity in the trichomes, and thus establishes a "head" and the "tail".[31] Trichomes usually reverse their polarity randomly with an average period on the order of minutes to hours.[32][33] Many species also form a semi-rigid sheath that is left behind as a hollow tube as the trichome moves forward. When the trichome reverses direction, it can move back into the sheath or break out.[34][2]

Oscillatoria is a genus of filamentous cyanobacterium named after the oscillation in its movement. Filaments in colonies slide back and forth against each other until the whole mass is reoriented to its light source. Oscillatoria is mainly blue-green or brown-green and is commonly found in watering-troughs. It reproduces by fragmentation forming long filaments of cells which can break into fragments called hormogonia. The hormogonia can then grow into new, longer filaments.
Häder's cyanograph experiment[]
In 1987, Häder demonstrated that trichomes can position themselves quite precisely within their environment through photomovement. In Häder's cyanograph experiment a photographic negative is projected onto a Petri dish containing a culture of Phormidium uncinatum.[35][36] After a few hours, the trichomes move away from the darker areas onto the lighter areas, forming a photographic positive on the culture. The experiment demonstrates that photomovement is effective not just for discrete light traps, but for minutely patterned, continuously differentiated light fields as well.[2]
See also[]
References[]
- ^ Whitton, Brian A.; Potts, Malcolm (2012). "Introduction to the Cyanobacteria". Ecology of Cyanobacteria II. pp. 1–13. doi:10.1007/978-94-007-3855-3_1. ISBN 978-94-007-3854-6.
- ^ a b c d e f g h i j k l Tamulonis, Carlos; Postma, Marten; Kaandorp, Jaap (2011). "Modeling Filamentous Cyanobacteria Reveals the Advantages of Long and Fast Trichomes for Optimizing Light Exposure". PLOS ONE. 6 (7): e22084. Bibcode:2011PLoSO...622084T. doi:10.1371/journal.pone.0022084. PMC 3138769. PMID 21789215.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ^ a b Stal LJ (2000) "Cyanobacterial Mats and Stromatolites". In
- ^ Claessen, Dennis; Rozen, Daniel E.; Kuipers, Oscar P.; Søgaard-Andersen, Lotte; Van Wezel, Gilles P. (2014). "Bacterial solutions to multicellularity: A tale of biofilms, filaments and fruiting bodies". Nature Reviews Microbiology. 12 (2): 115–124. doi:10.1038/nrmicro3178. hdl:11370/0db66a9c-72ef-4e11-a75d-9d1e5827573d. PMID 24384602. S2CID 20154495.
- ^ Nürnberg, Dennis J.; Mariscal, Vicente; Parker, Jamie; Mastroianni, Giulia; Flores, Enrique; Mullineaux, Conrad W. (2014). "Branching and intercellular communication in the Section V cyanobacterium Mastigocladus laminosus, a complex multicellular prokaryote". Molecular Microbiology. 91 (5): 935–949. doi:10.1111/mmi.12506. hdl:10261/99110. PMID 24383541. S2CID 25479970.
- ^ Herrero, Antonia; Stavans, Joel; Flores, Enrique (2016). "The multicellular nature of filamentous heterocyst-forming cyanobacteria". FEMS Microbiology Reviews. 40 (6): 831–854. doi:10.1093/femsre/fuw029. hdl:10261/140753. PMID 28204529.
- ^ Aguilera, Anabella; Klemenčič, Marina; Sueldo, Daniela J.; Rzymski, Piotr; Giannuzzi, Leda; Martin, María Victoria (2021). "Cell Death in Cyanobacteria: Current Understanding and Recommendations for a Consensus on Its Nomenclature". Frontiers in Microbiology. 12: 631654. doi:10.3389/fmicb.2021.631654. PMC 7965980. PMID 33746925.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ^ Risser DD, Chew WG, Meeks JC (April 2014). "Genetic characterization of the hmp locus, a chemotaxis-like gene cluster that regulates hormogonium development and motility in Nostoc punctiforme". Molecular Microbiology. 92 (2): 222–33. doi:10.1111/mmi.12552. PMID 24533832. S2CID 37479716.
- ^ Khayatan B, Bains DK, Cheng MH, Cho YW, Huynh J, Kim R, Omoruyi OH, Pantoja AP, Park JS, Peng JK, Splitt SD, Tian MY, Risser DD (May 2017). "A Putative O-Linked β-N-Acetylglucosamine Transferase Is Essential for Hormogonium Development and Motility in the Filamentous Cyanobacterium Nostoc punctiforme". Journal of Bacteriology. 199 (9): e00075–17. doi:10.1128/JB.00075-17. PMC 5388816. PMID 28242721.
- ^ Singh. Text Book of Botany Diversity of Microbes And Cryptogams. Rastogi Publications. ISBN 978-8171338894.
- ^ "Differences between Bacteria and Cyanobacteria". Microbiology Notes. 2015-10-29. Retrieved 2018-01-21.
- ^ Meeks JC, Elhai J, Thiel T, Potts M, Larimer F, Lamerdin J, Predki P, Atlas R (2001). "An overview of the genome of Nostoc punctiforme, a multicellular, symbiotic cyanobacterium". Photosynthesis Research. 70 (1): 85–106. doi:10.1023/A:1013840025518. PMID 16228364. S2CID 8752382.
- ^ Golden JW, Yoon HS (December 1998). "Heterocyst formation in Anabaena". Current Opinion in Microbiology. 1 (6): 623–9. doi:10.1016/s1369-5274(98)80106-9. PMID 10066546.
- ^ a b Fay P (June 1992). "Oxygen relations of nitrogen fixation in cyanobacteria". Microbiological Reviews. 56 (2): 340–73. doi:10.1128/MMBR.56.2.340-373.1992. PMC 372871. PMID 1620069.
- ^ Walsby AE (March 1994). "Gas vesicles". Microbiological Reviews. 58 (1): 94–144. doi:10.1128/MMBR.58.1.94-144.1994. PMC 372955. PMID 8177173.
- ^ Schapiro, Igor (May 2014). "Ultrafast photochemistry of Anabaena Sensory Rhodopsin: Experiment and theory". Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1837 (5): 589–597. doi:10.1016/j.bbabio.2013.09.014. PMID 24099700.
- ^ Basic Biology (18 March 2016). "Bacteria".
- ^ Wolk, C.P.; Ernst, A.; Elhai, J. (1994). Heterocyst metabolism and development. The Molecular Biology of Cyanobacteria. pp. 769–823. doi:10.1007/978-94-011-0227-8_27. ISBN 978-0-7923-3273-2.
- ^ a b Tamulonis, Carlos; Postma, Marten; Kaandorp, Jaap (2011). "Modeling Filamentous Cyanobacteria Reveals the Advantages of Long and Fast Trichomes for Optimizing Light Exposure". PLOS ONE. 6 (7): e22084. doi:10.1371/journal.pone.0022084. PMC 3138769. PMID 21789215.
- ^ a b Donkor, Victoria A.; Amewowor, Damina H.A.K.; Hã¤Der, Donat-P. (1993). "Effects of tropical solar radiation on the motility of filamentous cyanobacteria". FEMS Microbiology Ecology. 12 (2): 143–147. doi:10.1111/j.1574-6941.1993.tb00026.x.
- ^ Garcia-Pichel, Ferran; Mechling, Margaret; Castenholz, Richard W. (1994). "Diel Migrations of Microorganisms within a Benthic, Hypersaline Mat Community". Applied and Environmental Microbiology. 60 (5): 1500–1511. doi:10.1128/aem.60.5.1500-1511.1994. PMC 201509. PMID 16349251.
- ^ Fourã§Ans, Aude; Solã©, Antoni; Diestra, Ella; Ranchou-Peyruse, Anthony; Esteve, Isabel; Caumette, Pierre; Duran, Robert (2006). "Vertical migration of phototrophic bacterial populations in a hypersaline microbial mat from Salins-de-Giraud (Camargue, France)". FEMS Microbiology Ecology. 57 (3): 367–377. doi:10.1111/j.1574-6941.2006.00124.x. PMID 16907751.
- ^ Richardson, Laurie L.; Castenholz, Richard W. (1987). "Diel Vertical Movements of the Cyanobacterium Oscillatoria terebriformis in a Sulfide-Rich Hot Spring Microbial Mat". Applied and Environmental Microbiology. 53 (9): 2142–2150. doi:10.1128/aem.53.9.2142-2150.1987. PMC 204072. PMID 16347435.
- ^ McBride, Mark J. (2001). "Bacterial Gliding Motility: Multiple Mechanisms for Cell Movement over Surfaces". Annual Review of Microbiology. 55: 49–75. doi:10.1146/annurev.micro.55.1.49. PMID 11544349.
- ^ Reichenbach, H. (1981). "Taxonomy of the Gliding Bacteria". Annual Review of Microbiology. 35: 339–364. doi:10.1146/annurev.mi.35.100181.002011. PMID 6794424.
- ^ Hoiczyk, Egbert; Baumeister, Wolfgang (1998). "The junctional pore complex, a prokaryotic secretion organelle, is the molecular motor underlying gliding motility in cyanobacteria". Current Biology. 8 (21): 1161–1168. doi:10.1016/S0960-9822(07)00487-3. PMID 9799733. S2CID 14384308.
- ^ Hoiczyk, E. (2000). "Gliding motility in cyanobacteria: Observations and possible explanations". Archives of Microbiology. 174 (1–2): 11–17. doi:10.1007/s002030000187. PMID 10985737. S2CID 9927312.
- ^ Bhaya, D.; Watanabe, N.; Ogawa, T.; Grossman, A. R. (1999). "The role of an alternative sigma factor in motility and pilus formation in the cyanobacterium Synechocystis sp. Strain PCC6803". Proceedings of the National Academy of Sciences. 96 (6): 3188–3193. Bibcode:1999PNAS...96.3188B. doi:10.1073/pnas.96.6.3188. PMC 15917. PMID 10077659.
- ^ Hoiczyk, E.; Baumeister, W. (1995). "Envelope structure of four gliding filamentous cyanobacteria". Journal of Bacteriology. 177 (9): 2387–2395. doi:10.1128/jb.177.9.2387-2395.1995. PMC 176896. PMID 7730269.
- ^ Halfen, Lawrence N.; Castenholz, Richard W. (1971). "Gliding Motility in the Blue-Green Alga Oscillatoria Princeps 1". Journal of Phycology. 7 (2): 133–145. doi:10.1111/j.1529-8817.1971.tb01492.x. S2CID 86115246.
- ^ "Enhanced Model for Photophobic Responses of the Blue-Green Alga, <italic>Phormidium uncinatum</italic>". Plant and Cell Physiology. 1982. doi:10.1093/oxfordjournals.pcp.a076487.
- ^ Häder, Donat-P. (1987). "EFFECTS OF UV-B IRRADIATION ON PHOTOMOVEMENT IN THE DESMID, Cosmarium cucumis". Photochemistry and Photobiology. 46: 121–126. doi:10.1111/j.1751-1097.1987.tb04745.x. S2CID 97100233.
- ^ Gabai, V.L. (1985). "A one-instant mechanism of phototaxis in the cyanobacterium Phormidium uncinatum". FEMS Microbiology Letters. 30 (1–2): 125–129. doi:10.1111/j.1574-6968.1985.tb00998.x.
- ^ Häder, D.P. (1987) "Photomovement". The Cyanobacteria: 325-345.
- ^ Castenholz, Richard W. (2015-09-14), Cyanobacteria, Wiley, pp. 1–2, doi:10.1002/9781118960608.pbm00010, ISBN 9781118960608
- ^ Hangarter, Roger P.; Gest, Howard (2004). "Pictorial Demonstrations of Photosynthesis". Photosynthesis Research. Springer Science and Business Media LLC. 80 (1–3): 421–425. doi:10.1023/b:pres.0000030426.98007.6a. ISSN 0166-8595. PMID 16328838. S2CID 9453250.
- Cyanobacteria












