Cyanuric chloride

| |

| |
| Names | |
|---|---|
| IUPAC name
2,4,6-Trichloro-1,3,5-triazine
| |
| Other names
Trichlorotriazine
s-Triazine trichloride Cyanuryl chloride TCT | |
| Identifiers | |
3D model (JSmol)
|
|
| 124246 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.287 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 2670 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3Cl3N3 | |
| Molar mass | 184.40 g·mol−1 |
| Appearance | White powder |
| Odor | pungent |
| Density | 1.32 g/cm3 |
| Melting point | 144–148 °C (291–298 °F; 417–421 K) |
| Boiling point | 192 °C (378 °F; 465 K) |
| hydrolyzes | |
| Solubility in organic solvents | soluble |
| Solubility in THF | 0.34 g/mL |
| Solubility in CHCl3 | 0.17 g/mL |
| Structure | |
| monoclinic | |
| Hazards | |
| Safety data sheet | ICSC 1231 |
| GHS labelling: | |
  
| |
Signal word
|
Danger |
| H302, H314, H317, H330 | |
| P260, P261, P264, P270, P271, P272, P280, P284, P301+P312, P301+P330+P331, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P310, P320, P321, P330, P333+P313, P363, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | 
3
0
1 |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
485 mg/kg (rat, oral) |
| Related compounds | |
Related triazines
|
Cyanuric acid Cyanuric fluoride Cyanuric bromide Trichloroisocyanuric acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Cyanuric chloride is an organic compound with the formula (NCCl)3. This white solid is the chlorinated derivative of 1,3,5-triazine. It is the trimer of cyanogen chloride.[1] Cyanuric chloride is the main precursor to the popular but controversial herbicide atrazine.
Production[]
Cyanuric chloride is prepared in two steps from hydrogen cyanide via the intermediacy of cyanogen chloride, which is trimerized at elevated temperatures over a carbon catalyst:
- HCN + Cl2 → ClCN + HCl

In 2005, approximately 200,000 tons were produced.[2]
Industrial uses[]
It is estimated that 70% of cyanuric chloride is used in the preparation of the triazine-class pesticides, especially atrazine. Such reactions rely on the easy displacement of the chloride with nucleophiles such as amines:
- (ClCN)3 + 2 RNH2 → (RNHCN)(ClCN)2 + RNH3+Cl−
Other triazine herbicides, such as simazine, anilazine and cyromazine are made in an analogous way.[3]
Cyanuric chloride is also used as a precursor to dyes and crosslinking agents. The largest class of these dyes are the sulfonated triazine-stilbene optical brighteners (OBA) or fluorescent whitening agents (FWA) commonly found in detergent formulas and white paper.[2] Many reactive dyes also incorporate a triazine ring. They are also manufactured by way of the chloride displacement reaction shown above.[3][4]
Organic synthesis[]
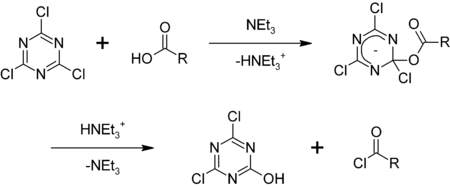
In one specialized application, cyanuric chloride is employed as a reagent in organic synthesis for the conversion of alcohols and carboxylic acids into alkyl and acyl chlorides, respectively:[5]
It is also used as a dehydrating agent and for the activation of carboxylic acids for reduction to alcohols. Heating with DMF gives "Gold's reagent" Me2NCH=NCH=NMe2+Cl−, which is a versatile source of aminoalkylations and a precursor to heterocycles.[6][7]
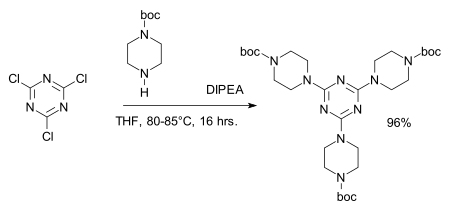
The chloride centers are easily replaced by amines to give melamine derivatives, for example in the synthesis of dendrimers:[8][9]
It is also employed the synthesis of an experimental adenosine receptor ligand.:[10]
Cyanuric chloride can also be used as an alternative to oxalyl chloride in the Swern oxidation.[11]
See also[]
- Thiazyl chloride trimer - structural analogue with sulfur atoms in-place of carbon
References[]
- ^ Cyanuric chloride at Chemicalland21.com
- ^ a b Klaus Huthmacher, Dieter Most "Cyanuric Acid and Cyanuric Chloride" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a08_191.
- ^ a b Ashford's Dictionary of Industrial Chemicals, 3rd edition, 2011, pages 2495-8
- ^ Tappe, Horst; Helmling, Walter; Mischke, Peter; Rebsamen, Karl; Reiher, Uwe; Russ, Werner; Schläfer, Ludwig; Vermehren, Petra (2000). Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a22_651. ISBN 978-3527306732.
- ^ K. Venkataraman & D. R. Wagle (1979). "Cyanuric chloride : a useful reagent for converting carboxylic acids into chlorides, esters, amides and peptides". Tetrahedron Lett. 20 (32): 3037–3040. doi:10.1016/S0040-4039(00)71006-9.
- ^ Probst, D. A.; Hanson, P. R.; Barda, D. A. "Cyanuric Chloride" in Encyclopedia of Reagents for Organic Synthesis, 2004, John Wiley & Sons. doi:10.1002/047084289X.rn00320
- ^ John T. Gupton; Steven A. Andrews (1990). "β-Dimethylaminomethylenation: N,N-Dimethyl-N'-p-tolylformamidine". Organic Syntheses.; Collective Volume, 7, p. 197
- ^ Abdellatif Chouai & Eric E. Simanek (2008). "Kilogram-Scale Synthesis of a Second-Generation Dendrimer Based on 1,3,5-Triazine Using Green and Industrially Compatible Methods with a Single Chromatographic Step". J. Org. Chem. 73 (6): 2357–2366. doi:10.1021/jo702462t. PMID 18307354.
- ^ Reagent: DIPEA, amine protective group: BOC
- ^ WO application 03101980, "1,3,5-TRIAZINE DERIVATIVES AS LIGANDS FOR HUMAN ADENOSINE-A3 RECEPTORS", published 2003-12-11 (Reagent number two: norephedrine, base DIPEA)
- ^ De Luca, L.; Giacomelli, G.; Procheddu, A (2001). "A Mild and Efficient Alternative to the Classical Swern Oxidation". J. Org. Chem. 66 (23): 7907–7909. doi:10.1021/jo015935s. PMID 11701058.
- Inorganic chlorine compounds
- Triazines
- Dehydrating agents