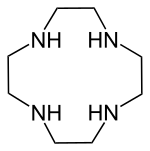
Cyclen
| |
| Names | |
|---|---|
| Preferred IUPAC name
1,4,7,10-Tetrazacyclododecane | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.102.391 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H20N4 | |
| Molar mass | 172.276 g·mol−1 |
| Appearance | White solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |

Crystal structure of a Zn(II) cation coordinated to cyclen and ethanol.[1]
Cyclen (1,4,7,10-tetraazacyclododecane) is a aza-crown ether with the formula (CH2CH2NH)4. It is a white solid.
Synthesis[]
High dilution conditions result in a low reaction rate penalty and this disadvantage is removed in an alternative procedure starting from triethylenetetraamine and dithiooxamide to a bisamidine – also a bis(imidazoline) – followed by reduction and ring expansion with DIBAL.[3]
In one study [4] cyclen is covalently bonded through a propylene to adenine and chelated with zinc diperchlorate. This complex is able to selectively bind uracil and uridine in a 1:2 ratio both through the adenine part and cyclen part of the molecule as evidenced by mass spectrometry.
See also[]
References[]
- ^ Schrodt, Antje; Neubrand, Anton; Van Eldik, Rudi (1997). "Fixation of CO2 by Zinc(II) Chelates in Alcoholic Medium. X-ray Structures of {[Zn(cyclen)]3(μ3-CO3)}(ClO4)4 and [Zn(cyclen)EtOH](ClO4)2". Inorg. Chem. 36 (20): 4579–4584. doi:10.1021/ic961368t. PMID 11670124.
- ^ Atkins, T. J.; Richman, J. E.; Oettle, W. F. (1978). "1,4,7,10,13,16-Hexaazacyclooctadecane". Org. Synth. 58: 86. doi:10.15227/orgsyn.058.0086.
- ^ Reed, David P.; Weisman, Gary R. (2002). "1,4,7,10-Tetraazacyclododecane". Org. Synth. 78: 73. doi:10.15227/orgsyn.078.0073.
- ^ Xia, Chuan-Qin; Tan, Xin-Yu; Chen, Shan-Yong; Yue, Yang; Yu, Xiao-Qi (2006). "The conjugate of adenine–cyclen Zn(II) complex: its synthesis and selective recognition abilities for uracil and uridine" (PDF). Arkivoc. 2: 68–76.
Further reading[]
- Suchý, M.; Hudson, R. H. E. (2008). "Synthetic Strategies Toward N-Functionalized Cyclens". Eur. J. Org. Chem. 2008 (29): 4847–4865. doi:10.1002/ejoc.200800636.
Categories:
- Ethyleneamines
- Macrocycles
- Chelating agents
- Nitrogen heterocycles
![Cyclen synthesis through a dilute ring closing reaction.[2]](http://upload.wikimedia.org/wikipedia/commons/thumb/6/60/Cyclen_synthesis.png/500px-Cyclen_synthesis.png)
