Cytochalasin E
| |
| Names | |
|---|---|
| Preferred IUPAC name
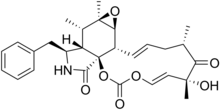
(4E,6R,8S,10E,11aS,11bS,12aR,13S,13aS,14S)-14-Benzyl-6-hydroxy-6,8,12a,13-tetramethyl-9,11a,11b,12a,13,13a,14,15-octahydro-2H-[1,3]dioxacyclotridecino[4,5-d]oxireno[2,3-f]isoindole-2,7,16(6H,8H)-trione | |
| Identifiers | |
3D model (JSmol)
|
|
| 3DMet | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.048.018 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C28H33NO7 | |
| Molar mass | 495.572 g·mol−1 |
| Density | 1.309 g/ml |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Toxic |
| GHS labelling: | |
 
| |
Signal word
|
Danger |
| H300, H310, H330, H361 | |
| P201, P202, P260, P262, P264, P270, P271, P280, P281, P284, P301+P310, P302+P350, P304+P340, P308+P313, P310, P320, P321, P322, P330, P361, P363, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Cytochalasin E, a member of the cytochalasin group, is an inhibitor of actin polymerization in blood platelets. It inhibits angiogenesis and tumor growth. Unlike and cytochalasin B, it does not inhibit glucose transport.
Because of its antiangiogenic effect, cytochalasin E is a potential drug for age-related macular degeneration, a kind of blindness caused by an abnormal proliferation of blood vessels in the eye.[2]
Cytochalasin E was found to be a potent and selective inhibitor of bovine capillary endothelial (BCE) cell proliferation. Cytochalasin E differs from other cytochalasin molecules by having an epoxide, which is required for specificity and potency. Cytochalasin E is a potent antiangiogenic agent that may be useful for treatments of cancer and other pathologic angiogenesis.[3]
References[]
- ^ Cytochalasin E from Aspergillus clavatus at Sigma-Aldrich
- ^ eyesight.org Archived 2006-05-19 at the Wayback Machine
- ^ Udagawa, T; Yuan, J; Panigrahy, D; Chang, YH; Shah, J; D'Amato, RJ (August 2000). "Cytochalasin E, an epoxide containing Aspergillus-derived fungal metabolite, inhibits angiogenesis and tumor growth". J. Pharmacol. Exp. Ther. 294: 421–7. PMID 10900214.
External pages[]
Cytochalasin E from Fermentek
Cytochalasin E from Cayman Chemical
- Mycotoxins
- Actin inhibitors
- Carbonate esters
- Epoxides
- Lactones
- Biochemistry stubs