Dechlorane plus

| |
| Names | |
|---|---|
| IUPAC name
1,2,3,4,7,8,9,10,13,13,14,14-dodecachloro-1,4,4a,5,6,6a,7,10,10a,11,12,12a-dodecahydro-1,4,7,10-dimethanodibenzo[a,e]cyclooctene
| |
| Other names
Dechloran A
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.033.575 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C18H12Cl12 | |
| Molar mass | 653.72 g/mol |
| Appearance | white, crystalline solid |
| Density | 1.8 g/cm3 |
| Melting point | 350 °C (662 °F; 623 K) (decomposes) |
| 0.044–249 μg/L | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Dechlorane plus (abbrev. DDC-CO[1]) is a polychlorinated flame retardant produced by Oxychem. Its log P is 11.27±0.94.[2] It is produced by the Diels-Alder reaction of two equivalents of hexachlorocyclopentadiene with one equivalent of cyclooctadiene. The syn and anti isomer are formed in the approximate ratio of 1:3.[3][4]
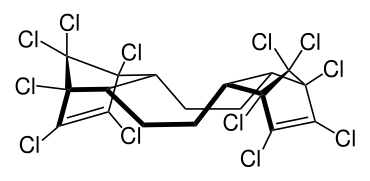
syn isomer
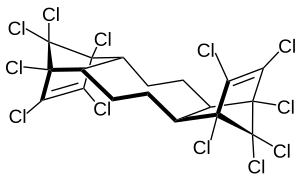
anti isomer
Dechlorane plus was first found in the environment in 2006 in air around the Great Lakes.[5] Since then, its environmental occurrence has been further documented in several studies including sediments of the Great Lakes;[6] zooplankton, fish, and mussels in Lake Winnipeg and Lake Ontario;[7] air and seawater from the Arctic to Antarctica;[8] and Chinese air.[9] Modeling data indicates that Dechlorane Plus may be persistent, bioaccumulative, and subject to long-range transport and that it may be candidates for Annex D evaluation under the United Nations Stockholm Convention on Persistent Organic Pollutants.[10]
Dechlorane plus was added to the list of REACH Substances of Very High Concern on January 15, 2018.[11]
Literature[]
- ENVIRON Health Sciences Institute. "Dechlorane Plus High Production Volume (HPV) Chemical Challenge Program Test Plan. 201-15635" (PDF). Archived from the original (PDF) on 2012-10-23.
References[]
- ^ Bergman, Å.; Rydén, A.; Law, R. J.; De Boer, J.; Covaci, A.; Alaee, M.; Birnbaum, L.; Petreas, M.; Rose, M.; Sakai, S.; Van Den Eede, N.; Van Der Veen, I. (2012). "A novel abbreviation standard for organobromine, organochlorine and organophosphorus flame retardants and some characteristics of the chemicals". Environment International. 49: 57–82. doi:10.1016/j.envint.2012.08.003. PMC 3483428. PMID 22982223.
- ^ "1,6,7,8,9,14,15,16,17,17,18,18-dodecachloropent... - Registration Dossier - ECHA". echa.europa.eu. Retrieved 2020-03-18.
- ^ Sverko, E.; Tomy, G. T.; Reiner, E. J.; Li, Y. F.; McCarry, B. E.; Arnot, J. A.; Law, R. J.; Hites, R. A. (2011). "Dechlorane Plus and Related Compounds in the Environment: A Review". Environmental Science & Technology. 45 (12): 5088–5098. Bibcode:2011EnST...45.5088S. doi:10.1021/es2003028. PMID 21574656.
- ^ Garcia, J. G.; Fronczek, F. R.; McLaughlin, M. L. (1991). "Tandem reverse-electron-demand diels-alder reactions of 1,5-cyclooctadiene". Tetrahedron Letters. 32 (28): 3289–3292. doi:10.1016/S0040-4039(00)92688-1.
- ^ Hoh, Eunha; Lingyan Zhu; Ronald A. Hites (2006). "Dechlorane Plus, a chlorinated flame retardant, in the Great Lakes". Environmental Science & Technology. 40 (4): 1184–1189. Bibcode:2006EnST...40.1184H. doi:10.1021/es051911h. PMID 16572773.
- ^ Sverko, Ed; Gregg T. Tomy; Chris H. Marvin; Donna Zaruk; Eric Reiner; Paul A. Helm; Brad Hill; Brian E. McCarry (2007). "Dechlorane plus levels in sediment of the lower Great Lakes". Environmental Science & Technology. 42 (2): 361–366. doi:10.1021/es0710104. PMID 18284131.
- ^ Tomy, Gregg; Kerri Pleskach; Nargis Ismail; D. Michael Whittle; Paul A. Helm; E. D. Sverko; Donna Zaruk; Chris H. Marvin (2007). "Isomers of dechlorane plus in Lake Winnipeg and Lake Ontario food webs". Environmental Science & Technology. 41 (7): 2249–2254. Bibcode:2007EnST...41.2249T. doi:10.1021/es062781v. PMID 17438771.
- ^ Möller, Axel; Zhiyong Xie; Renate Sturm; Ralf Ebinghaus (2010). "Large-scale distribution of Dechlorane Plus in air and seawater from the Arctic to Antarctica". Environmental Science & Technology. 44 (23): 8977–8982. Bibcode:2010EnST...44.8977M. doi:10.1021/es103047n. PMID 21047104.
- ^ Ren, Nanqi; Ed Sverko; Yi-Fan Li; Zhi Zhang; Tom Harner; Degao Wang; Xinnan Wan; Brian E. McCarry (2008). "Levels and isomer profiles of Dechlorane Plus in Chinese air". Environmental Science & Technology. 42 (17): 6476–6480. Bibcode:2008EnST...42.6476R. doi:10.1021/es800479c. PMID 18800517.
- ^ Sverko, Ed; Gregg T. Tomy; Eric J. Reiner; Yi-Fan Li; Brian E. McCarry; Jon A. Arnot; Robin J. Law; Ronald A. Hites (2011). "Dechlorane plus and related compounds in the environment: a review". Environmental Science & Technology. 45 (12): 5088–5098. Bibcode:2011EnST...45.5088S. doi:10.1021/es2003028. PMID 21574656.
- ^ "1,6,7,8,9,14,15,16,17,17,18,18-Dodecachloropentacyclo[12.2.1.16,9.02,13.05,10]octadeca-7,15-diene ("Dechlorane Plus"™) - Candidate List of substances of very high concern for Authorisation - ECHA". European Chemicals Agency. ECHA. Retrieved January 16, 2018.
- Organochlorides

