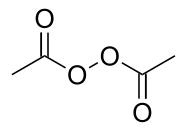
Diacetyl peroxide
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Acetic peroxyanhydride[1] | |
| Other names
acetylperoxide; diacetyl peroxide; Peroxide, diacetyl; ethanoyl peroxide; acetyl ethaneperoxoate; ethanoyl ethaneperoxoate; peracetic acid acetyl ester
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.003.409 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 2084 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H6O4 | |
| Molar mass | 118.088 g·mol−1 |
| Appearance | Colorless crystals[2] |
| Density | 1.163 g/cm3[2] |
| Melting point | 30 °C (86 °F; 303 K) |
| Boiling point | 121.4 °C (250.5 °F; 394.5 K) at 760 mmHg; 63 °C (145 °F; 336 K) at 21 mmHg[3] |
| slight in cold water [2] | |
| Hazards | |
| Main hazards | Explosive |
| NFPA 704 (fire diamond) | 
1
2
4 |
| Flash point | 32.2 °C (90.0 °F; 305.3 K) (45 °C [113 °F; 318 K][4]) |
| Explosive data | |
| Shock sensitivity | Very high / moderate when wet |
| Friction sensitivity | Very high / moderate when wet |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Diacetyl peroxide is the organic peroxide with the formula (CH3CO2)2. It is a white solid or oily liquid with a sharp odor.[4] Since the pure material poses an explosion hazard, it is often used as a solution, e.g., in dimethyl phthalate as a solvent.[2]
Preparation[]
Diacetyl peroxide forms upon combining hydrogen peroxide and excess acetic anhydride. Peracetic acid is an intermediate.[5]
Safety[]
Organic peroxides are typically explosive since they contain both the oxidizer, the O-O bond, and reducing agents, the C-C and C-H bonds.[6]
It is shock sensitive and explosive.[7][8][2]
The threshold quantity for Process Safety Management per Occupational Safety and Health Administration 1910.119 is 5,000 lb (2,300 kg) if the concentration of the diacetyl peroxide solution is greater than 70%.[9]
There have been reports of detonation of the pure material. The 25% solution also has explosive potential.[10] The crystalline peroxide is especially shock sensitive and a high explosion risk.[11][8]
Health hazards[]
Contact with liquid causes irritation of eyes and skin. If ingested, irritates mouth and stomach.[12][13][14][15]
References[]
- ^ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 829. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ^ a b c d e Lewis, R. J., Sr, ed. (1997). Hawley's Condensed Chemical Dictionary (13th ed.). New York, NY: John Wiley & Sons. p. 13.
- ^ Lide, D. R., ed. (1998–1999). CRC Handbook of Chemistry and Physics (79th ed.). Boca Raton, Florida: CRC Press. pp. 3–250.
- ^ a b "Acetyl peroxide" (PDF). NJ.gov.
- ^ "Chemical Safety: Synthesis Procedure". Chemical & Engineering News. 89 (2): 2. 2011-01-10.
- ^ Sax, N. I. (1975). Dangerous Properties of Industrial Materials (4th ed.). New York, NY: Van Nostrand Reinhold. p. 357.
- ^ "Chemical Safety: Synthesis Procedure". Chemical & Engineering News. 89 (2): 2. 2011-01-10.
- ^ a b National Fire Protection Association (1978). Fire Protection Guide on Hazardous Materials (7th ed.). Boston, MA: National Fire Protection Association. pp. 49–110.
- ^ [1]
- ^ National Research Council (1981). Prudent Practices for Handling Hazardous Chemicals in Laboratories. Washington, DC: National Academy Press. p. 106.
- ^ Bretherick, L. (1990). Handbook of Reactive Chemical Hazards (4th ed.). Boston, MA: Butterworth-Heinemann. pp. 453, 1104.
- ^ National Research Council (1981). Prudent Practices for Handling Hazardous Chemicals in Laboratories. Washington, DC: National Academy Press. p. 106.
- ^ International Labour Office (1998). Encyclopaedia of Occupational Health and Safety. 1–4 (4th ed.). Geneva: International Labour Office. p. 104.349.
- ^ Clayton, G.D.; Clayton, F.E., eds. (1993–1994). Patty's Industrial Hygiene and Toxicology. 2A–2F (4th ed.). New York, NY: John Wiley & Sons. p. 545.
- ^ Mackison, F. W.; Stricoff, R. S.; Partridge, L. J., Jr, eds. (Jan 1981). NIOSH/OSHA - Occupational Health Guidelines for Chemical Hazards. DHHS(NIOSH) Publication No. 81–123. Washington, DC: U.S. Government Printing Office. p. 1.
- "Peroxide, diacetyl (C4H6O4)". Landolt-Boernstein Substance/Property Index.
- "Zuordnung der Organischen Peroxide zu Gefahrgruppen nach § 3 Abs. 1". Berufsgenossenschaft Handel und Warendistribution (in German).
- Organic peroxides
- Explosive chemicals
- Liquid explosives
- Organic peroxide explosives
- Carbonyl compounds