Dihydrogen phosphate
| |
| Names | |
|---|---|
| IUPAC name
Dihydrogen Phosphate
| |
| Systematic IUPAC name
Phosphoric acid, ion(1-) | |
| Other names
Phosphoric acid, ion(1-)
Dehydrophosphoric acid (1-)
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| H2O4P−1 | |
| Molar mass | 96.986 g·mol−1 |
| Conjugate acid | Phosphoric Acid |
| Related compounds | |
Related compounds
|
Phosphate, Monohydrogen phosphate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
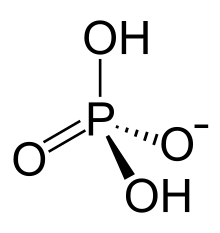
Dihydrogen phosphate or dihydrogenphosphate ion is an inorganic ion with the formula [H2PO4]−. Phosphates occur widely in natural systems.[1]
These sodium phosphates are artificially used in food processing and packaging as emulsifying agents, neutralizing agents, surface-activating agents, and leavening agents providing humans with benefits. Emulsifying agents prevent separation of two ingredients in processed foods that would separate under natural conditions while neutralizing agents make processed foods taste fresher longer and lead to an increased shelf-life of these foods.[1] Surface-activating agents prevent surface-tension formation on liquid-containing processed foods and finally, leavening agents are used in processed foods to aid in the expansion of yeast in baked goods.[1]
Dihydrogen phosphate is employed in the production of pharmaceuticals furthering their importance to medical practitioners of Gastroenterology and humans in general. In this medical discipline, sodium phosphates are used as natural laxatives.[1] Other medical applications include using sodium and potassium phosphates along with other medications to increase their therapeutic effects. Inflammation, certain cancers, and ulcers can benefit from the use of combination therapy with sodium and potassium phosphates.[1]
Potassium dihydrogen phosphate, the potassium salt, is useful to human in the form of pesticides. Potassium dihydrogen phosphate is a fungicide that is used to prevent powdery mildew on many fruits.[2] Fruits that can benefit from the addition of potassium dihydrogen phosphate includes common fruits, peppers, and roses.[2]
Structure[]
The dihydrogen phosphate anion consists of a central phosphorus atom surrounded by 2 equivalent oxygen atoms and 2 hydroxy groups in a tetrahedral arrangement.[3] Dihydrogen phosphate can be identified as an anion, an ion with an overall negative charge, with dihydrogen phosphates being a negative 1 charge.[3] Dihydrogen phosphate contains 4 H bond acceptors and 2 H bond donors,[3] and has 0 rotatable bonds.[4]
Acid-base equilibria[]
Dihydrogen phosphate is an intermediate in the multistep conversion of the polyprotic phosphoric acid to phosphate:[5]
This multistep conversion exemplifies that the dihydrogen phosphate ion is the conjugate base to phosphoric acid, while also acting as the conjugate acid to the phosphate ion.[3] This means that dihydrogen phosphate can be both a hydrogen donor and acceptor.
| Equilibrium | Disassociation constant, pKa[6] |
|---|---|
| H3PO4 ⇌ H 2PO− 4 + H+ |
pKa1 = 2.14[a] |
| H 2PO− 4 ⇌ HPO2− 4 + H+ |
pKa2 = 7.20 |
| HPO2− 4 ⇌ PO3− 4 + H+ |
pKa3 = 12.37 |
Examples[]
- Monocalcium phosphate (Ca(H2PO4)2)
- Ammonium dihydrogen phosphate ((NH4)(H2PO4))
Safety[]
Many foods including milk, eggs, poultry, and nuts contain these sodium phosphates.[1]
Notes[]
- ^ Values are at 25 °C and 0 ionic strength.
References[]
- ^ a b c d e f Tech, Noah. "Sodium Phosphates: From Food to Pharmacology | Noah Technologies". info.noahtech.com. Retrieved 2021-03-23.
- ^ a b "Document Display | NEPIS | US EPA". nepis.epa.gov. Retrieved 2021-03-23.
- ^ a b c d PubChem. "Dihydrogen phosphate". pubchem.ncbi.nlm.nih.gov. Retrieved 2021-03-23.
- ^ "dihydrogenphosphate | H2O4P | ChemSpider". www.chemspider.com. Retrieved 2021-03-23.
- ^ "Phosphoric Acid H3PO4". www.aqion.de. Retrieved 2021-03-23.
- ^ Powell, Kipton J.; Brown, Paul L.; Byrne, Robert H.; Gajda, Tamás; Hefter, Glenn; Sjöberg, Staffan; Wanner, Hans (2005). "Chemical speciation of environmentally significant heavy metals with inorganic ligands. Part 1: The Hg2+, Cl−, OH−, CO2−
3, SO2−
4, and PO3−
4 aqueous systems". Pure Appl. Chem. 77 (4): 739–800. doi:10.1351/pac200577040739.
- Ions
- Phosphates