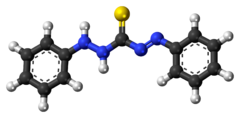
Dithizone

| |
| |
| Names | |
|---|---|
| IUPAC name
(1E)-3-anilino-1-phenylimino-thiourea
| |
| Other names
Diphenylthiocarbazone,
1,5-Diphenylthiocarbazone
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.000.413 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C13H12N4S | |
| Molar mass | 256.33 g·mol−1 |
| Hazards | |
| Safety data sheet (SDS) | External MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Dithizone is a sulfur-containing organic compound. It is a good ligand, and forms complexes with many metals such as lead and mercury.
Dithizone may be prepared by reacting phenylhydrazine with carbon disulfide, followed by reaction with potassium hydroxide.[1]
Dithizone is used to assess the purity of human pancreatic islet preparations used for transplantation into patients with type 1 diabetes. Dithizone binds zinc ions present in the islet's beta cells, and therefore stains the islets red. Exocrine tissue also present in the preparations does not bind dithizone, and is therefore not stained.[2]
Dithizone
Dithizone in Ethanol 96%
References[]
- ^ John H. Billman and Elizabeth S. Cleland (1955). "Dithizone". Organic Syntheses.; Collective Volume, 3, p. 360
- ^ Ricordi, C; Gray, DW; Hering, BJ; Kaufman, DB; Warnock, GL; Kneteman, NM; Lake, SP; London, NJ; et al. (1990). "Islet isolation assessment in man and large animals" (PDF). Acta Diabetologica Latina. 27 (3): 185–95. doi:10.1007/BF02581331. PMID 2075782.
Categories:
- Thiocarbonyl compounds

