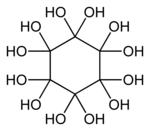
Dodecahydroxycyclohexane
| |

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Cyclohexanedodecol | |
| Other names
Dodecahydroxycyclohexane
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6O12H12 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Dodecahydroxycyclohexane is an organic compound with molecular formula C6O12H12 or C6(OH)12. It is a sixfold geminal diol with a cyclohexane backbone and can be regarded as a sixfold hydrate of cyclohexanehexone (C6O6).
Dihydrate[]
The dihydrate C6O12H12·2H2O can be crystallized from methanol as colorless plates or prisms, that decomposes at about 100 °C.[1]

This compound was synthetized by [2] in 1862 by oxidation of benzenehexol C6(OH)6 or tetrahydroxy-p-benzoquinone C6(OH)4O2 and characterized by R. Nietzki and others in 1885,[3] although the product was for a long time assumed to be hexaketocyclohexane with water of crystallization (C6O6·8H2O).
Indeed, this product is still commonly marketed as cyclohexanehexone octahydrate, hexaketocyclohexane octahydrate, triquinoyl octahydrate and similar names. Its true nature was suspected since the 1950s or earlier,[4] but was confirmed by X-ray diffraction analysis only in 2005.[5]
See also[]
References[]
- ^ Alexander J. Fatiadi; Horace S. Isbell; William F. Sager (March–April 1963). "Cyclic Polyhydroxy Ketones. I. Oxidation Products of Hexahydroxybenzene (Benzenehexol)" (PDF). Journal of Research of the National Bureau of Standards Section A. 67A (2): 153–162. doi:10.6028/jres.067A.015. PMC 6640573. PMID 31580622. Archived from the original (PDF) on 2009-03-25. Retrieved 2009-03-22.
- ^ Jos. Ud. Lerch (1862). "Ueber Kohlenoxydkalium und die aus demselben darstellbaren Säuren". Journal für Praktische Chemie. 87 (1): 427–469. doi:10.1002/prac.18620870146.
- ^ R. Nietzki, Th. Benckiser (1885). "Ueber Hexaoxybenzolderivate und ihre Beziehungen zur Krokonsäure und Rhodizonsäure". Berichte der Deutschen Chemischen Gesellschaft. 18 (1): 499–515. doi:10.1002/cber.188501801110.
- ^ Willis B. Person and Dale G. Williams (1957). "Infrared spectra and the structures of leuconic acid and triquinoyl". J. Phys. Chem. 61 (7): 1017–1018. doi:10.1021/j150553a047.
- ^ Thomas M. Klapötke; Kurt Polborn; Jan J. Weigand (March 2005). "Dodecahydroxycyclohexane dihydrate" (PDF). Acta Crystallographica E. Archived from the original (PDF) on 2020-03-04.
- Cyclitols
- Cyclohexanols
- Geminal diols