Electrochemical reduction of carbon dioxide
The electrochemical reduction of carbon dioxide is the conversion of carbon dioxide (CO2) to more reduced chemical species using electrical energy. It is one possible step in the broad scheme of carbon capture and utilization, nevertheless it is deemed to be one of the most promising approaches.[1]
Electrochemical reduction of carbon dioxide represents a possible means of producing chemicals or fuels, converting carbon dioxide (CO2) to organic feedstocks such as formic acid (HCOOH),[2] carbon monoxide (CO), methane (CH4), ethylene (C2H4) and ethanol (C2H5OH).[3][4][5] Among the more selective metallic catalysts in this field are tin for formic acid, silver for carbon monoxide and copper for methane, ethylene or ethanol. Methanol, propanol and 1-butanol have also been produced via CO2 electrochemical reduction, albeit in small quantities.[6]
The first examples of electrochemical reduction of carbon dioxide are from the 19th century, when carbon dioxide was reduced to carbon monoxide using a zinc cathode. Research in this field intensified in the 1980s following the oil embargoes of the 1970s. As of 2021, pilot-scale carbon dioxide electrochemical reduction is being developed by several companies, including Siemens,[7] Dioxide Materials,[8] and Twelve.[9] The techno-economic analysis was recently conducted to assess the key technical gaps and commercial potentials of the carbon dioxide electrolysis technology at near ambient conditions.[10][11]
Chemicals from carbon dioxide[]
In carbon fixation, plants convert carbon dioxide into sugars, from which many biosynthetic pathways originate. The catalyst responsible for this conversion, RuBisCO, is the most common protein on earth. Some anaerobic organisms employ enzymes to convert CO2 to carbon monoxide, from which fatty acids can be made.[12]
In industry, a few products are made from CO2, including urea, salicylic acid, methanol, and certain inorganic and organic carbonates.[13] In the laboratory, carbon dioxide is sometimes used to prepare carboxylic acids in a process known as carboxylation. No electrochemical CO2 electrolyzer that operates at room temperature has been commercialized. Elevated temperature solid oxide electrolyzer cells (SOECs) for CO2 reduction to CO are commercially available. For example, Haldor Topsoe offers SOECs for CO2 reduction with a reported 6-8 kWh per Nm3[note 1] CO produced and purity up to 99.999% CO.[14]
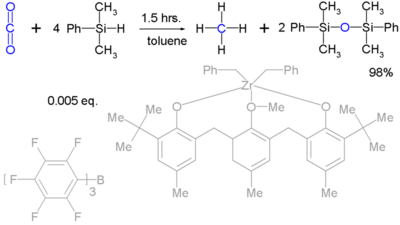
Hydrosilanes reduce carbon dioxide (to methane):[15] Unfortunately such reactions are stoichiometric and exclusively of academic interest.
Electrocatalysis[]
The electrochemical reduction of carbon dioxide to various products is usually described as:
| Reaction | Reduction Potential[16]
Eo (V) |
|---|---|
| CO2 + 2 H+ + 2 e− → HCOOH | −0.61 |
| CO2 + 2 H+ + 2 e− → CO + H2O | −0.53 |
| CO2 + 8 H+ + 8 e− → CH4 + 2 H2O | −0.24 |
| 2 CO2 + 12 H+ + 12 e− → C2H4 + 4 H2O | −0.349 |
| 2 CO2 + 12 H+ + 12 e− → C2H5OH + 3 H2O | −0.329 |
The redox potentials for these reactions are similar to that for hydrogen evolution in aqueous electrolytes, thus electrochemical reduction of CO2 is usually competitive with hydrogen evolution reaction.[5]
Electrochemical methods have gained significant attention: 1) at ambient pressure and room temperature; 2) in connection with renewable energy sources (see also solar fuel) 3) competitive controllability, modularity and scale-up are relatively simple.[17] The electrochemical reduction or electrocatalytic conversion of CO2 can produce value-added chemicals such methane, ethylene, ethanol, etc., and the products are mainly dependent on the selected catalysts and operating potentials (applying reduction voltage).[18][19][20]
A variety of homogeneous and heterogeneous catalysts[21] have been evaluated.[3][5] Many such processes are assumed to operate via the intermediacy of metal carbon dioxide complexes.[22] Many processes suffer from high overpotential, low current efficiency, low selectivity, slow kinetics, and/or poor catalyst stability.[23]
The composition of the electrolyte can be decisive.[24][25] Gas-diffusion electrodes are beneficial.[26][27][28]
See also[]
- Electromethanogenesis
- Biobattery
- Electrofuel
- Lemon battery
- Photoelectrochemical reduction of carbon dioxide
- Photochemical reduction of carbon dioxide
- Electrochemical energy conversion
- Bioelectrochemical reactor
Notes[]
- ^ Normal Cubic Meter - the quantity of gas that occupies one cubic meter at standard temperature and pressure.
References[]
- ^ "Dream or Reality? Electrification of the Chemical Process Industries". www.aiche-cep.com. Retrieved 2021-08-22.
- ^ Valenti G, Melchionna M, Montini T, Boni A, Nasi L, Fonda E, et al. (2020). "Water-Mediated ElectroHydrogenation of CO2 at Near-Equilibrium Potential by Carbon Nanotubes/Cerium Dioxide Nanohybrids". ACS Appl. Energy Mater. 3 (9): 8509–8518. doi:10.1021/acsaem.0c01145.
- ^ a b Centi G, Perathoner S (2009). "Opportunities and prospects in the chemical recycling of carbon dioxide to fuels". Catalysis Today. 148 (3–4): 191–205. doi:10.1016/j.cattod.2009.07.075.
- ^ Qiao J, Liu Y, Hong F, Zhang J (January 2014). "A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels". Chemical Society Reviews. 43 (2): 631–75. doi:10.1039/c3cs60323g. PMID 24186433.
- ^ a b c Appel AM, Bercaw JE, Bocarsly AB, Dobbek H, DuBois DL, Dupuis M, et al. (August 2013). "Frontiers, opportunities, and challenges in biochemical and chemical catalysis of CO2 fixation". Chemical Reviews. 113 (8): 6621–58. doi:10.1021/cr300463y. PMC 3895110. PMID 23767781.
- ^ Ting LR, García-Muelas R, Martín AJ, Veenstra FL, Chen ST, Peng Y, et al. (November 2020). "Electrochemical Reduction of Carbon Dioxide to 1-Butanol on Oxide-Derived Copper". Angewandte Chemie. 59 (47): 21072–21079. doi:10.1002/anie.202008289. PMC 7693243. PMID 32706141.
- ^ "CO2 is turned into feedstock". siemens-energy.com Global Website. Retrieved 2021-07-04.
- ^ "CO2 Electrolyzers With Record Performance". Dioxide Materials. Retrieved 2021-07-04.
- ^ Masel, Richard I.; Liu, Zengcai; Yang, Hongzhou; Kaczur, Jerry J.; Carrillo, Daniel; Ren, Shaoxuan; Salvatore, Danielle; Berlinguette, Curtis P. (2021). "An industrial perspective on catalysts for low-temperature CO 2 electrolysis". Nature Nanotechnology. 16 (2): 118–128. Bibcode:2021NatNa..16..118M. doi:10.1038/s41565-020-00823-x. ISSN 1748-3395. OSTI 1756565. PMID 33432206. S2CID 231580446.
- ^ Jouny, Matthew; Luc, Wesley; Jiao, Feng (2018-02-14). "General Techno-Economic Analysis of CO2 Electrolysis Systems". Industrial & Engineering Chemistry Research. 57 (6): 2165–2177. doi:10.1021/acs.iecr.7b03514. ISSN 0888-5885. OSTI 1712664.
- ^ Shin, Haeun; Hansen, Kentaro U.; Jiao, Feng (October 2021). "Techno-economic assessment of low-temperature carbon dioxide electrolysis". Nature Sustainability. 4 (10): 911–919. doi:10.1038/s41893-021-00739-x. ISSN 2398-9629. S2CID 235801320.
- ^ Fontecilla-Camps JC, Amara P, Cavazza C, Nicolet Y, Volbeda A (August 2009). "Structure-function relationships of anaerobic gas-processing metalloenzymes". Nature. 460 (7257): 814–22. Bibcode:2009Natur.460..814F. doi:10.1038/nature08299. PMID 19675641. S2CID 4421420.
- ^ Susan Topham, "Carbon Dioxide" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a05_165
- ^ "Produce Your Own Carbon Monoxide - on-site and on-demand". www.topsoe.com. Haldor Topsoe. Archived from the original on 28 February 2021.
- ^ From Carbon Dioxide to Methane: Homogeneous Reduction of Carbon Dioxide with Hydrosilanes Catalyzed by Zirconium-Borane Complexes Tsukasa Matsuo and Hiroyuki Kawaguchi J. Am. Chem. Soc.; 2006; 128, pp 12362 - 12363; doi:10.1021/ja0647250
- ^ Sun Z, Ma T, Tao H, Fan Q, Han B (2017). "Fundamentals and Challenges of Electrochemical CO2 Reduction Using Two-Dimensional Materials". Chem. 3 (4): 560–587. doi:10.1016/j.chempr.2017.09.009.
- ^ Lee S, Lee J (February 2016). "Electrode Build-Up of Reducible Metal Composites toward Achievable Electrochemical Conversion of Carbon Dioxide". ChemSusChem. 9 (4): 333–44. doi:10.1002/cssc.201501112. PMID 26610065.
- ^ Lee S, Ju H, Machunda R, Uhm S, Lee JK, Lee HJ, Lee J (2015). "Sustainable production of formic acid by electrolytic reduction of gaseous carbon dioxide". Journal of Materials Chemistry A. 3 (6): 3029–3034. doi:10.1039/C4TA03893B.
- ^ Whipple DT, Kenis PJ (2010). "Prospects of CO2 Utilization via Direct Heterogeneous Electrochemical Reduction". The Journal of Physical Chemistry Letters. 1 (24): 3451–3458. doi:10.1021/jz1012627.
- ^ Machunda RL, Ju H, Lee J (2011). "Electrocatalytic reduction of CO2 gas at Sn-based gas diffusion electrode". Current Applied Physics. 11 (4): 986–988. Bibcode:2011CAP....11..986M. doi:10.1016/j.cap.2011.01.003.
- ^ Hori Y (2008). "Electrochemical CO2 Reduction on Metal Electrodes". Modern Aspects of Electrochemistry. Modern Aspects of Electrochemistry. Vol. 42. pp. 89–80. doi:10.1007/978-0-387-49489-0_3. ISBN 978-0-387-49488-3.
- ^ Benson EE, Kubiak CP, Sathrum AJ, Smieja JM (January 2009). "Electrocatalytic and homogeneous approaches to conversion of CO2 to liquid fuels". Chemical Society Reviews. 38 (1): 89–99. doi:10.1039/b804323j. PMID 19088968. S2CID 20705539.
- ^ Halmann MM, Steinberg M (May 1998). Greenhouse gas carbon dioxide mitigation: science and technology. CRC press. ISBN 1-56670-284-4.
- ^ Rosen BA, Salehi-Khojin A, Thorson MR, Zhu W, Whipple DT, Kenis PJ, Masel RI (November 2011). "Ionic liquid-mediated selective conversion of CO₂ to CO at low overpotentials". Science. 334 (6056): 643–4. Bibcode:2011Sci...334..643R. doi:10.1126/science.1209786. PMID 21960532. S2CID 31774347.
- ^ Service RF (1 September 2017). "Two new ways to turn 'garbage' carbon dioxide into fuel". Science Magazine. doi:10.1126/science.aap8497.
- ^ Thorson MR, Siil KI, Kenis PJ (2013). "Effect of Cations on the Electrochemical Conversion of CO 2 to CO". Journal of the Electrochemical Society. 160 (1): F69–F74. doi:10.1149/2.052301jes. ISSN 0013-4651. S2CID 95111100.
- ^ Lv JJ, Jouny M, Luc W, Zhu W, Zhu JJ, Jiao F (December 2018). "A Highly Porous Copper Electrocatalyst for Carbon Dioxide Reduction". Advanced Materials. 30 (49): e1803111. doi:10.1002/adma.201803111. PMID 30368917.
- ^ Dinh CT, Burdyny T, Kibria MG, Seifitokaldani A, Gabardo CM, García de Arquer FP, et al. (May 2018). "CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface". Science. 360 (6390): 783–787. doi:10.1126/science.aas9100. PMID 29773749.
Further reading[]
- LaConti AB, Molter TM, Zagaja JA (May 1986). Electrochemical Reduction of Carbon Dioxide. Online: Information for the Defense Industry. Archived from the original on 27 March 2012.
- Fujita E (January 2000). Carbon Dioxide (Reduction). Upton, NY (United States): Brookhaven National Lab. (BNL).
- Neelameggham NR. "Carbon Dioxide Reduction Technologies: A Synopsis of the Symposium at TMS 2008". The Minerals, Metals & Materials Society (TMS).
- Carbon dioxide
- Energy engineering
- Electrochemical engineering