Elexacaftor/tezacaftor/ivacaftor
| Combination of | |
|---|---|
| Elexacaftor | Cystic fibrosis transmembrane conductance regulator (CFTR) corrector |
| Tezacaftor | CFTR corrector |
| Ivacaftor | Chloride channel opener |
| Clinical data | |
| Trade names | Trikafta, Kaftrio |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a619061 |
| License data | |
| Pregnancy category | |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| KEGG | |
Elexacaftor/tezacaftor/ivacaftor, sold under the brand names Trikafta (US) and Kaftrio (Europe), is a fixed-dose combination medication used with adults and children older than age 6 that have cystic fibrosis with a f508del mutation or other mutations.[5] Patients with cystic fibrosis have thicker mucus secretions due to genetic mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) protein which inhibits the transfer of chloride and sodium ions from cells. Elexacaftor/tezacaftor/ivacaftor is composed of a combination of ivacaftor, a chloride channel opener, and elexacaftor and tezacaftor, CFTR modulators.[5]
It is approved use in the United States for people aged 6 years of age or older with the f508del mutation or 177 other cystic fibrosis mutations.[6] It is also approved for use in Canada, the European Union and Australia.[7][8][9]
Cystic Fibrosis and CFTR[]
Cystic fibrosis is an autosomal recessive genetic disorder of the CFTR protein which reduces chloride and sodium ion transport through the cell membrane, causing thicker than normal mucus secretions.[10][11] The CFTR protein is found in epithelial cells of the lung, liver, pancreas, digestive tract, and reproductive tracts.[12][13][14] CFTR has a role in the production of mucus, sweat, and digestive fluids.[15] The thickened mucus can lead to inflammation, respiratory infections, and clogged ducts.[16][17][18]
Pharmacology[]
Effects[]
A phase III trial showed people treated with elexacaftor/tezacaftor/ivacaftor improved in FEV1 at four weeks with sustained improvement at 24 weeks. Rate of pulmonary exacerbation was 63% lower and sweat chloride concentration was 41.8 mmol/L lower.[19][20][21] It's effectiveness is dependent on the type of CF mutations the patient has.[22]
Mechanism of Action[]
Elexacaftor/tezacaftor/ivacaftor is a tridrug treatment in which the medications work together to increase the transport of chloride and sodium ions, and reducing thick mucus production.[23]

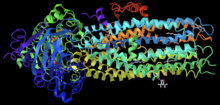

CFTR Channel Potentiator[]
Ivacaftor is a selective small-molecule potentiator of the CFTR protein that increases the protein's ability to open chloride channels.[24][25] Its effectiveness is highly dependent on the amount of CFTR protein at the cell surface and the responsiveness of the mutant CFTR protein.[26] Ivacaftor's primary target is to treat class III CFTR gating mutations like G551D as well as other less common mutations.[25] In the crystalline figure, you can see ivacaftor, shown as a gray ball and stick model on the bottom-right, bound to CFTR docked in a cleft formed by transmembrane helices at the protein-lipid interface.[27]
CFTR Correctors[]


Elexacaftor and Tezacaftor act as CFTR correctors to repair F508del processing by binding to the CFTR protein to increase the availability of CFTR protein on the cell surface.[28] They work by modulating the position of the CFTR protein into the right position on the cell surface.[29]
The combination of increased CFTR protein in the correct position on the cell surface with ivacaftor's potentiation of chloride channel opening results in increased transport of chloride and thinned mucus secretions.[30]

Formulations[]
Trikafta utilizes a combination tablet containing 100 mg of elexacaftor, 50 mg of tezacaftor, and 75 mg of ivacaftor, and a separate tablet containing 150mg of ivacaftor.[31]
Recommended Dosage[]
The morning dose is two combination tablets containing elexacaftor 100 mg, tezacaftor 50 mg and ivacaftor 75 mg. The evening dose is one ivacaftor 150mg tablet.[32]
Side/Adverse Effects[]
The most common side effects affecting more than 5% of patients are headache, upper respiratory tract infection, abdominal pain, diarrhea, rash, alanine aminotransferase increased, nasal congestion, blood creatine phosphokinase increased, aspartate aminotransferase increased, rhinorrhea, rhinitis, influenza, sinusitis and blood bilirubin increased.[31][33]
Interactions[]
Concomitant use with CYP3A inducers is not recommended.[34][35] Dosage must be adjusted with moderate or strong CYP3A inhibitors.[35][34]
Other drugs with the potential for interaction include: warfarin, digoxin, statins, glyburide, nateglinide, repaglinide.[35][34]
Pharmacokinetics[]
Elexacaftor/tezacaftor/ivacaftor is primarily metabolized by CYP3A4/5. This medication should be taken with a high fat meal to improve absorption through the gut. [36] It is excreted as metabolites or unchanged mainly through feces and to a smaller extent urine. The mean effective half-life of elexacaftor, tezacaftor, and ivacaftor is 27.4 hours, 25.1 hours, and 15 hours, respectively. [31]
Research[]
| Trial | Type | Primary Endpoint | Target age | Target Mutations | Results | References |
|---|---|---|---|---|---|---|
| Trial 1 | A placebo-controlled trial in patients heterozygous for the F508del mutation and another specific mutation | Absolute change in ppFEV1 from baseline at Week 4 | People aged 12 years and older | • Heterozygous for the F508del mutation and one of ~200 other mutations in the CFTR gene that resulted in either: - No CFTR protein - A CFTR protein that lacks baseline activity and is not responsive to ivacaftor and tezacaftor/ivacaftor • ppFEV1 between 40% to 90% at screening | percentage of predicted FEV1 that was 13.8 points higher at 4 weeks and 14.3 points higher through 24 weeks | [37][38] |
| Trial 2 | A double-blind, active-controlled, phase 3 study | Absolute change in ppFEV1 from baseline at Week 4 | People aged 12 years and older | Homozygous for the F508del mutation | Elexacaftor/tezacaftor/ivacaftor showed improvements in percent predicted forced expiratory volume (ppFEV1) over patients receiving tezacaftor/ivacaftor | [39] |
| Trial 3 | Open label study with no placebo control | Safety, pharmacokinetics and efficacy | Children aged 6-11 | Homozygous for the F508del mutation OR
- Heterozygous for the F508del mutation and one of ~200 other mutations in the CFTR gene that resulted in either: • No CFTR protein • A CFTR protein that lacks baseline activity and is not responsive to ivacaftor and tezacaftor/ivacaftor |
safety and pharmacokinetic profiles were generally consistent with those observed in older patients | [40] |
CFTR mutations that are responsive to elexacaftor/tezacaftor/ivacaftor were determined by an in-vitro study of Fischer Rat Thyroid (FRT) cells that expressed mutant CFTR. Elexacaftor/tezacaftor/ivacaftor showed effectiveness with mutations where the CFTR protein was being successfully delivered to the cell surface.[3]
Society and culture[]
Legal status[]
United States[]
The combination was approved for use in the United States in 2019 for people twelve years and older with cystic fibrosis who have at least one F508del mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, which is estimated to represent 90% of the cystic fibrosis population.[41] In December 2020, after an additional clinical trial was completed, and FDA approval was expanded for 177 other cystic fibrosis mutations.[6] FDA approval for children aged 6-11 was added in January 2021 after third clinical trial was completed.[42]
The U.S. Food and Drug Administration (FDA) granted the application priority review, in addition to fast track, breakthrough therapy, and orphan drug designations. The drug's manufacturer Vertex Pharmaceuticals will receive a rare pediatric disease priority review voucher for having developed this therapy.[43]
Australia[]
On March 30, 2021 health regulators in Australia approved trikafta for patients aged 12 years and older with at least one copy of the F508del mutation.[44]
Canada[]
In June of 2020, Health Canada approved Trikafta for patients ages 12 and up.[8] In September 2021, the provinces Alberta and Saskatchewan announced they will join Ontario in funding the medication. They will determine coverage on a case-by-case basis using a criteria that has not yet been announced.[45]
European Union[]
In June 2020, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) recommended its approval for the treatment of cystic fibrosis.[46][47] It was approved for medical use in the European Union in August 2020.[4]
Cost[]
The list price of a year's treatment in the US is US$311,000.[48] However, a 2020 report by Institute for Clinical and Economic Review found that the price has made the treatment not cost effective and that "an appropriate health-benefit price would range from $67,900–$85,500 per year".[49][50]
Spain[]
On November 19, 2021 the Spanish government approved the reimbursement of KAFTRIO (ivacaftor/tezacaftor/elexacaftor) for patients ages 12 and older with at least one copy of the F508del mutation.[51]
References[]
- ^ a b "Trikafta". Therapeutic Goods Administration (TGA). April 8, 2021. Retrieved September 8, 2021.
- ^ a b "AusPAR: Elexacaftor/tezacaftor/ivacaftor and ivacaftor". Therapeutic Goods Administration (TGA). July 8, 2021. Retrieved September 8, 2021.
- ^ a b "Trikafta- elexacaftor, tezacaftor, and ivacaftor kit". DailyMed. January 29, 2020. Retrieved August 22, 2020.
- ^ a b "Kaftrio EPAR". European Medicines Agency (EMA). June 23, 2020. Retrieved August 21, 2020.
- ^ a b "Trikafta- elexacaftor, tezacaftor, and ivacaftor kit". DailyMed. January 29, 2020. Retrieved August 22, 2020.
- ^ a b "FDA Approves Expansion of Modulators for People With Certain Rare Mutations". Cystic Fibrosis Foundation. Retrieved October 3, 2021.
- ^ "Kaftrio EPAR". European Medicines Agency (EMA). June 23, 2020. Retrieved August 21, 2020.
- ^ a b Wexler M. "Health Canada Approves Trikafta for Eligible CF Patients, 12 and Up". Retrieved November 8, 2021.
- ^ Australian Government Department of Health Therapeutic Goods Administration (July 8, 2021). "AusPAR: Elexacaftor/tezacaftor/ivacaftor and ivacaftor". Therapeutic Goods Administration (TGA). Retrieved November 28, 2021.
- ^ O'Sullivan BP, Freedman SD (May 2009). "Cystic fibrosis". Lancet. 373 (9678): 1891–1904. doi:10.1016/s0140-6736(09)60327-5. PMID 19403164. S2CID 46011502.
- ^ Hodson M, Geddes D, Bush A, eds. (2012). Cystic Fibrosis (3rd ed.). London: Hodder Arnold. p. 3. ISBN 978-1-4441-1369-3. Archived from the original on September 8, 2017.
- ^ Sharma S, Hanukoglu I (April 2019). "Mapping the sites of localization of epithelial sodium channel (ENaC) and CFTR in segments of the mammalian epididymis". Journal of Molecular Histology. 50 (2): 141–154. doi:10.1007/s10735-019-09813-3. PMID 30659401. S2CID 58026884.
- ^ Sharma S, Hanukoglu A, Hanukoglu I (April 2018). "Localization of epithelial sodium channel (ENaC) and CFTR in the germinal epithelium of the testis, Sertoli cells, and spermatozoa". Journal of Molecular Histology. 49 (2): 195–208. doi:10.1007/s10735-018-9759-2. PMID 29453757. S2CID 3761720.
- ^ Enuka Y, Hanukoglu I, Edelheit O, Vaknine H, Hanukoglu A (March 2012). "Epithelial sodium channels (ENaC) are uniformly distributed on motile cilia in the oviduct and the respiratory airways". Histochemistry and Cell Biology. 137 (3): 339–353. doi:10.1007/s00418-011-0904-1. PMID 22207244. S2CID 15178940.
- ^ Buckingham L (2012). Molecular Diagnostics: Fundamentals, Methods and Clinical Applications (2nd ed.). Philadelphia: F.A. Davis Co. p. 351. ISBN 978-0-8036-2975-2. Archived from the original on September 8, 2017.
- ^ Flume PA, Mogayzel PJ, Robinson KA, Rosenblatt RL, Quittell L, Marshall BC (August 2010). "Cystic fibrosis pulmonary guidelines: pulmonary complications: hemoptysis and pneumothorax". American Journal of Respiratory and Critical Care Medicine. 182 (3): 298–306. doi:10.1164/rccm.201002-0157OC. PMID 20675678.
- ^ Mitchell RS, Kumar V, Robbins SL, et al. (2007). Robbins Basic Pathology. Saunders/Elsevier. p. 1253, 1254. ISBN 978-1-4160-2973-1.
- ^ Mitchell RS, Kumar V, Robbins SL, et al. (2007). Robbins Basic Pathology. Saunders/Elsevier. p. 1253, 1254. ISBN 978-1-4160-2973-1.
- ^ Middleton PG, Mall MA, Dřevínek P, Lands LC, McKone EF, Polineni D, et al. (VX17-445-102 Study Group) (November 2019). "Elexacaftor-Tezacaftor-Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele". The New England Journal of Medicine. 381 (19): 1809–1819. doi:10.1056/NEJMoa1908639. PMC 7282384. PMID 31697873.
- ^ Taylor-Cousar JL, Mall MA, Ramsey BW, McKone EF, Tullis E, Marigowda G, et al. (April 2019). "Clinical development of triple-combination CFTR modulators for cystic fibrosis patients with one or two F508del alleles". ERJ Open Research. 5 (2): 00082–2019. doi:10.1183/23120541.00082-2019. PMC 6571452. PMID 31218221.
- ^ Clinical trial number NCT03525444 for "A Phase 3 Study of VX-445 Combination Therapy in Subjects With Cystic Fibrosis Heterozygous for the F508del Mutation and a Minimal Function Mutation (F/MF)" at ClinicalTrials.gov
- ^ Tice JA, Kuntz KM, Wherry K, Seidner M, Rind DM, Pearson SD (February 2021). "The effectiveness and value of novel treatments for cystic fibrosis". Journal of Managed Care & Specialty Pharmacy. 27 (2): 276–280. doi:10.18553/jmcp.2021.27.2.276. ISSN 2376-0540. PMID 33506736.
- ^ PubChem. "Tezacaftor". pubchem.ncbi.nlm.nih.gov. Retrieved November 7, 2021.
- ^ "PharmGKB". PharmGKB. Retrieved December 9, 2021.
- ^ a b Zaher A, ElSaygh J, Elsori D, ElSaygh H, Sanni A (July 2021). "A Review of Trikafta: Triple Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Modulator Therapy". Cureus. 13 (7): e16144. doi:10.7759/cureus.16144. PMC 8266292. PMID 34268058.
- ^ PubChem. "Ivacaftor". pubchem.ncbi.nlm.nih.gov. Retrieved November 7, 2021.
- ^ Liu F, Zhang Z, Levit A, Levring J, Touhara KK, Shoichet BK, Chen J (June 2019). "Structural identification of a hotspot on CFTR for potentiation". Science. 364 (6446): 1184–1188. doi:10.1126/science.aaw7611. PMC 7184887. PMID 31221859.
- ^ Zaher A, ElSaygh J, Elsori D, ElSaygh H, Sanni A (July 2021). "A Review of Trikafta: Triple Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Modulator Therapy". Cureus. 13 (7): e16144. doi:10.7759/cureus.16144. PMC 8266292. PMID 34268058.
- ^ PubChem. "Tezacaftor". pubchem.ncbi.nlm.nih.gov. Retrieved November 7, 2021.
- ^ Zaher A, ElSaygh J, Elsori D, ElSaygh H, Sanni A (July 2021). "A Review of Trikafta: Triple Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Modulator Therapy". Cureus. 13 (7): e16144. doi:10.7759/cureus.16144. PMC 8266292. PMID 34268058.
- ^ a b c "TRIKAFTA". . February 8, 2021. Archived from the original on August 12, 2020. Retrieved October 13, 2021.
- ^ "TRIKAFTA". . February 8, 2021. Archived from the original on August 12, 2020. Retrieved October 13, 2021.
- ^ "Trikafta Side Effects". Drugs.com. January 24, 2021. Archived from the original on October 3, 2021. Retrieved October 3, 2021.
- ^ a b c "elexacaftor/tezacaftor/ivacaftor (Rx)". Medscape. Archived from the original on October 28, 2020. Retrieved October 3, 2021.
- ^ a b c Vertex Pharmaceuticals. Trikafta (Elexacaftor/tezacaftor/ivacaftor) [package insert]. U.S. Food and Drug Administration website.https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/212273s004lbl.pdf. Revised June 2021. Accessed October 3, 2021.
- ^ "PharmGKB". PharmGKB. Retrieved December 9, 2021.
- ^ Middleton PG, Mall MA, Dřevínek P, Lands LC, McKone EF, Polineni D, et al. (November 2019). "Elexacaftor-Tezacaftor-Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele". The New England Journal of Medicine. 381 (19): 1809–1819. doi:10.1056/NEJMoa1908639. PMC 7282384. PMID 31697873.
- ^ Lopes-Pacheco M (2020). "CFTR Modulators: The Changing Face of Cystic Fibrosis in the Era of Precision Medicine". Frontiers in Pharmacology. 10: 1662. doi:10.3389/fphar.2019.01662. PMC 7046560. PMID 32153386.
- ^ Heijerman HG, McKone EF, Downey DG, Van Braeckel E, Rowe SM, Tullis E, et al. (November 2019). "Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial". Lancet. 394 (10212): 1940–1948. doi:10.1016/S0140-6736(19)32597-8. PMC 7571408. PMID 31679946.
- ^ Zemanick ET, Taylor-Cousar JL, Davies J, Gibson RL, Mall MA, McKone EF, et al. (June 2021). "A Phase 3 Open-Label Study of Elexacaftor/Tezacaftor/Ivacaftor in Children 6 through 11 Years of Age with Cystic Fibrosis and at Least One F508del Allele". American Journal of Respiratory and Critical Care Medicine. 203 (12): 1522–1532. doi:10.1164/rccm.202102-0509OC. PMC 8483230. PMID 33734030.
- ^ "FDA approves new breakthrough therapy for cystic fibrosis". U.S. Food and Drug Administration (FDA) (Press release). October 21, 2019. Archived from the original on November 13, 2019. Retrieved November 13, 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "FDA Accepts Vertex Application for Expansion of Trikafta to Include Children ages 6-11". www.cff.org. Bethesda, MD: Cystic Fibrosis Foundation. Retrieved October 3, 2021.
- ^ "FDA approves new breakthrough therapy for cystic fibrosis". U.S. Food and Drug Administration (FDA) (Press release). October 21, 2019. Archived from the original on November 13, 2019. Retrieved November 13, 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Maia M. "Trikafta Approved in Australia for CF Patients Starting at Age 12". Retrieved November 28, 2021.
- ^ Wexler M. "CF Therapy Trikafta Now Covered in 2 More Canadian Provinces". Retrieved November 8, 2021.
- ^ "New medicine for cystic fibrosis patients". European Medicines Agency (EMA) (Press release). June 26, 2020. Retrieved June 26, 2020.
- ^ "Elexacaftor + ivacaftor + tezacaftor". SPS - Specialist Pharmacy Service. July 28, 2020. Retrieved August 21, 2020.
- ^ Maddipatla M, O'Donnell C (October 21, 2019). "Vertex prices cystic fibrosis combo treatment at $311,000-per-year". Reuters. Retrieved October 23, 2019.
- ^ Martins I. "Trikafta Very Effective CF Therapy, But Still Too Costly, ICER Reports". Retrieved August 21, 2020.
- ^ "Modulator Treatments for Cystic Fibrosis: Effectiveness and Value" (PDF). Institute for Clinical and Economic Review. Institute for Clinical and Economic Review. April 27, 2020. Retrieved August 21, 2020.
- ^ "Vertex Announces Reimbursement Agreement in Spain for KAFTRIO® (ivacaftor/tezacaftor/elexacaftor) in Combination With Ivacaftor to Treat People With Cystic Fibrosis 12 Years and Older With At Least One F508del Mutation in the CFTR Gene - ANSA.it". www.ansa.it. Retrieved November 28, 2021.
External links[]
- "Elexacaftor". Drug Information Portal. U.S. National Library of Medicine.
- "Ivacaftor regimen with Tezacaftor". Drug Information Portal. U.S. National Library of Medicine.
- Breakthrough therapy
- Combination drugs
- Cystic fibrosis
- Orphan drugs