Enol


Enols, or more formally, alkenols, are a type of reactive structure or intermediate in organic chemistry that is represented as an alkene (olefin) with a hydroxyl group attached to one end of the alkene double bond. The terms enol and alkenol are portmanteaus deriving from "-ene"/"alkene" and the "-ol" suffix indicating the hydroxyl group of alcohols, dropping the terminal "-e" of the first term. Generation of enols often involves removal of a hydrogen adjacent (α-) to the carbonyl group—i.e., deprotonation, its removal as a proton, H+. When this proton is not returned at the end of the stepwise process, the result is an anion termed an enolate (see images at right). The enolate structures shown are schematic; a more modern representation considers the molecular orbitals that are formed and occupied by electrons in the enolate. Similarly, generation of the enol often is accompanied by "trapping" or masking of the hydroxy group as an ether, such as a silyl enol ether.[2]
In organic chemistry, keto–enol tautomerism refers to a chemical equilibrium between a keto form (a ketone or an aldehyde) and an enol (an alcohol). The keto and enol forms are said to be tautomers of each other. The interconversion of the two forms involves the movement of an alpha hydrogen atom and the reorganisation of bonding electrons; hence, the isomerism qualifies as tautomerism.[3]
Enolization[]
Organic esters, ketones, and aldehydes with an α-hydrogen (C-H bond adjacent to the carbonyl group) often form enols. The reaction involves migration of a proton from carbon to oxygen:[2]
- RC(O)CHR'2 RC(OH)=CR'2
In the case of ketones, the conversion is called a keto-enol tautomerism, although this name is often more generally applied to all such tautomerizations. Usually the equilibrium constant is so small that the enol is undetectable spectroscopically.
In some compounds with two (or more) carbonyls, the enol form becomes dominant. The behavior of 2,4-pentanedione illustrates this effect:[4]
| carbonyl | enol | Kenolization |
|---|---|---|
| CH3CHO | CH2=CHOH | 5.8 x 10−7 |
| CH3C(O)CH3 | CH3C(OH)=CH2 | 5.12 x 10−7 |
| CH3CO2CH3 | CH2=CH(OH)OCH3 | 4x10−20 |
| C6H5C(O)CH3 | C6H5C(OH)=CH2 | 1 x 10−8 |
| CH3C(O)CH2C(O)CH3 | CH3C(O)CH=C(OH)CH3 | 0.27 |
Enols are derivatives of vinyl alcohol, with a C=C-OH connectivity. Deprotonation of organic carbonyls gives the enolate anion, which are a strong nucleophile. A classic example for favoring the keto form can be seen in the equilibrium between vinyl alcohol and acetaldehyde (K = [enol]/[keto] ≈ 3 × 10−7). In 1,3-diketones, such as acetylacetone (2,4-pentanedione), the enol form is favored.
The acid-catalyzed conversion of an enol to the keto form proceeds by proton transfer from O to carbon. The process does not occur intramolecularly, but requires participation of solvent or other mediators.
Stereochemistry of ketonization[]
If R1 and R2 (note equation at top of page) are different substituents, there is a new stereocenter formed at the alpha position when an enol converts to its keto form. Depending on the nature of the three R groups, the resulting products in this situation would be diastereomers or enantiomers.
Enediols[]
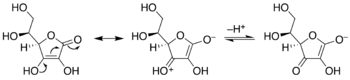
Enediols are alkenes with a hydroxyl group on each carbon of the C=C double bond. Normally such compounds are disfavored components in equilibria with acyloins. One special case is catechol, where the C=C subunit is part of an aromatic ring. In some other cases however, enediols are stabilized by flanking carbonyl groups. These stabilized enediols are called reductones. Such species are important in glycochemistry, e.g., the Lobry de Bruyn-van Ekenstein transformation.[6]
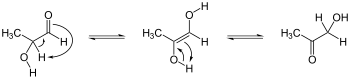 Keto-enediol tautomerizations. Enediol in the center; acyloin isomers at left and right. Ex. is hydroxyacetone, shown at right.
Keto-enediol tautomerizations. Enediol in the center; acyloin isomers at left and right. Ex. is hydroxyacetone, shown at right.
Phenols[]
Phenols represent a kind of enol. For some phenols and related compounds, the keto tautomer plays an important role. Many of the reactions of resorcinol involve the keto tautomer, for example. Naphthalene-1,4-diol exists in observable equilibrium with the diketone tetrahydronaphthalene-1,4-dione.[7]
Biochemistry[]
Keto–enol tautomerism is important in several areas of biochemistry.
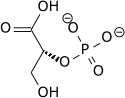
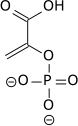
The high phosphate-transfer potential of phosphoenolpyruvate results from the fact that the phosphorylated compound is "trapped" in the less thermodynamically favorable enol form, whereas after dephosphorylation it can assume the keto form.
The enzyme enolase catalyzes the dehydration of 2-phosphoglyceric acid to the enol phosphate ester. Metabolism of PEP to pyruvic acid by pyruvate kinase (PK) generates adenosine triphosphate (ATP) via substrate-level phosphorylation.[8]
|
|

| ||||
| H2O | ADP | ATP | ||||

|

| |||||
| H2O | ||||||
Reactivity[]
The terminus of the double bond in enols is nucleophilic. Its reactions with electrophilic organic compounds underlies the tremendous importance of enol-based intermediates in a wide array of important life processes (i.e., in biochemistry, as intermediates in enzyme-catalysed reactions), as well as being central to modern synthetic organic chemistry (e.g., in applications of aldol and related reactions).
Enolates[]
Deprotonation of enolizable ketones, aldehydes, and esters gives enolates.[9][10] Enolates can be trapped by the addition of electrophiles at oxygen. Silylation gives silyl enol ether.[11] Acylation gives esters such as vinyl acetate.[12]
Stable enols[]
In general, enols are less stable than their keto equivalents, because of the favorability of the C=O double bond over C=C double bond. However, enols can be stabilized kinetically or thermodynamically.
Kinetically stable enols[]
Kinetically stable enols revert very slowly to keto form. The keto form is thermodynamically favored, but the enol survives long enough to be kinetically stable. Like enol formation, ketone formation from enol is catalyzed by acid or base. Without either, the enol form has a somewhat long lifetime. For example, heating ethylene glycol to 900°C at low pressure will yield vinyl alcohol, the enol of acetaldehyde. Acetaldehyde is still preferred over the enol form, but without a catalyst, formation moves very slowly.


Enols can also be stable if it is very difficult for the carbon atom to be protonated. Steric hindrance can block a protonating agent and without protonation, the ketone cannot be formed. In the example to the right, the two benzene rings shield the enol from proton attack, so the enol is kinetically stable.[13]
Thermodynamically stable enols[]
Thermodynamically stable enols are stabilized by delocalization of the enol and resonance. We can create them by adding functional groups that allow for these effects. Some 1,3-dicarbonyl compounds are relatively stable in enol form. With the (1,3) arrangement, the enols are conjugated with the two carbonyl groups. Through tautomerization, the two enols are in rapid equilibrium. Another stabilizing factor in 1,3-dicarbonyls is intramolecular hydrogen bonding.[14] We can see both of these factors in acetylacetone. The intramolecular hydrogen bond creates a favorable six-membered ring.


In addition, if formation of an enol creates a conjugated resonance system, the increased resonance is also more favorable and can stabilize the enol. In the example below, in ketone form, the two rings cannot form one resonance system, but the enol form make the entire molecule a fully conjugated system, adding more resonance and stability.

The most stable enols are phenols. The enol form is stabilized by aromaticity, which substantially outweighs the favorability of a C=O bond has over a C=C bond. As a result, these molecules solely exist in phenol form, and the "ketone" of phenol is not observed. [13]
See also[]
- Alkenal
- Enolase
- Ketone
- Ynol
- Geminal diol, another form of ketones and aldehydes in water solutions
- Regioselectivity
References[]
- ^ Caminati, W.; Grabow, J.-U. (2006). "The C2v Structure of Enolic Acetylacetone". J. Am. Chem. Soc. 128 (3): 854–857. doi:10.1021/ja055333g. PMID 16417375.
- ^ Jump up to: a b Smith MB, March J (2001). Advanced Organic Chemistry (5th ed.). New York: Wiley Interscience. pp. 1218–1223. ISBN 0-471-58589-0.
- ^ Clayden, Jonathan; Greeves, Nick; Warren, Stuart (2012). Organic chemistry (2nd ed.). New York: Oxford University Press. pp. 450–451. ISBN 978-0-19-927029-3.
- ^ Manbeck, Kimberly A.; Boaz, Nicholas C.; Bair, Nathaniel C.; Sanders, Allix M. S.; Marsh, Anderson L. (2011). "Substituent Effects on Keto–Enol Equilibria Using NMR Spectroscopy". J. Chem. Educ. 88 (10): 1444–1445. Bibcode:2011JChEd..88.1444M. doi:10.1021/ed1010932.
- ^ Guthrie, J. Peter; Povar, Igor (2013). "Equilibrium constants for enolization in solution by computation alone". Journal of Physical Organic Chemistry. 26 (12): 1077–1083. doi:10.1002/poc.3168.
- ^ Schank, Kurt (1972). "Reductones". Synthesis. 1972 (4): 176–90. doi:10.1055/s-1972-21845.
- ^ Kündig, E. Peter; Enríquez García, Alvaro; Lomberget, Thierry; Bernardinelli, Gérald (2006). "Rediscovery, Isolation, and Asymmetric Reduction of 1,2,3,4-Tetrahydronaphthalene-1,4-dione and Studies of Its [Cr(CO)3] Complex". Angewandte Chemie International Edition. 45 (1): 98–101. doi:10.1002/anie.200502588. PMID 16304647.
- ^ Berg, Jeremy M.; Tymoczko, Stryer (2002). Biochemistry (5th ed.). New York: W.H. Freeman and Company. ISBN 0-7167-3051-0.
- ^ Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, ISBN 978-0-471-72091-1
- ^ Manfred Braun (2015). Modern Enolate Chemistry: From Preparation to Applications in Asymmetric Synthesis. Wiley‐VCH. doi:10.1002/9783527671069. ISBN 9783527671069.
- ^ Mukaiyama, T.; Kobayashi, S. Org. React. 1994, 46, 1. doi:10.1002/0471264180.or046.01
- ^ G. Roscher (2007). "Vinyl Esters". Ullmann's Encyclopedia of Chemical Technology. Weinheim: Wiley-VCH. doi:10.1002/14356007.a27_419. ISBN 978-3527306732.
- ^ Jump up to: a b Clayden, Jonathan (2012). Organic Chemistry. Oxford University Press. pp. 456–459.
- ^ Zhou, Yu-Qiang; Wang, Nai-Xing; Xing, Yalan; Wang, Yan-Jing; Hong, Xiao-Wei; Zhang, Jia-Xiang; Chen, Dong-Dong; Geng, Jing-Bo; Dang, Yanfeng; Wang, Zhi-Xiang (2013-01-14). "Stable acyclic aliphatic solid enols: synthesis, characterization, X-ray structure analysis and calculations". Scientific Reports. 3 (1): 1058. Bibcode:2013NatSR...3E1058Z. doi:10.1038/srep01058. ISSN 2045-2322. PMC 3544012. PMID 23320139.
External links[]
| Wikiquote has quotations related to: Enol |
- Biosynthesis
- Functional groups
- Metabolism
- Reactive intermediates
- Alcohols
- Alkene derivatives
- Enols
- Organic reactions


