Enthalpy of sublimation
This article needs additional citations for verification. (January 2021) |
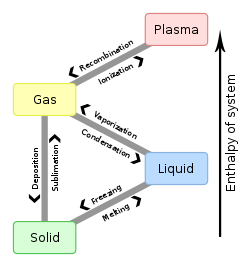
The enthalpy of sublimation, or heat of sublimation, is the heat required to change one mole of a substance from solid state to gaseous state at a given combination of temperature and pressure, usually standard temperature and pressure (STP). The heat of sublimation is usually expressed in kJ/mol, although the less customary kJ/kg is also encountered.
Sublimation enthalpies[]
| symbol | substances | Sublimation enthalpy (kJ/mol) |
|---|---|---|
| Na | sodium | 108 |
| K | potassium | 89 |
| Rb | rubidium | 82 |
| Cs | caesium | 70 |
| Mg | magnesium | 15 |
| Ca | calcium | 192 |
| Sr | strontium | 164 |
| Ba | barium | 176 |
| I2 | iodine | 62.4[1] |
| C10H8 | naphthalene | 72.9[1] |
| CO2 | carbon dioxide | 25[1] |
See also[]
- Heat
- Sublimation (chemistry)
- Phase transition
- Clausius-Clapeyron equation
References[]
- ^ a b c Chickos, James S.; Acree, William E. (2002). "Enthalpies of Sublimation of Organic and Organometallic Compounds. 1910–2001". Journal of Physical and Chemical Reference Data. 31 (2): 537–698. doi:10.1063/1.1475333. ISSN 0047-2689.
Categories:
- Enthalpy