Ferroxyl indicator solution
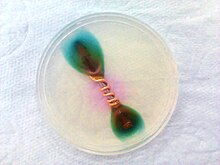
Corrosion of iron nail with coiled copper in medium with ferroxyl indicator
Ferroxyl indicator is a solution containing potassium hexacyanoferrate(III) and phenolphthalein. It turns blue in the presence of Fe2+ ions, pink in the presence of hydroxide ions, it can be used to detect metal oxidation. It is often used to detect rusting in various situations.
It can be prepared by dissolving 10g sodium chloride and 1g potassium hexacyanoferrate(III) in distilled water, adding 10 cm3 phenolphthalein indicator, then making up to 500 cm3 with distilled water.[1]
References[]
- ^ "SALTERS ADVANCED CHEMISTRY A2 TECHNICIANS MANUAL" (PDF). benjamin-mills.com.
Categories:
- Chemical tests
- Corrosion