Flubber (material)

Flubber (named from the film The Absent-Minded Professor), Glorp, Glurch, or Slime is a rubbery polymer formed by cross-linking of polyvinyl alcohol (PVA) with a boron compound. Flubber can be made by combining polyvinyl-acetate-based adhesives with baking soda, borax, and (optionally) shaving cream as an elementary science education experiment.[1]
Reaction[]
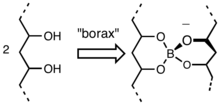
The gelation process entails formation of a borate ester that crosslinks the chains of the PVA.[2] Borate esters form readily by condensation of hydroxyl groups and the B-OH groups.[3]
Properties[]
Flubber is a non-Newtonian fluid that flows under low stress, but breaks under higher stresses and pressures. This combination of fluid-like and solid-like properties makes it a Maxwell fluid. Its behavior can also be described as being viscoplastic or gelatinous.
See also[]
- Silly Putty
- Gunge
- Nickelodeon compounds
- Slime (toy)
References[]
- ^ Parratore, Phil. Wacky Science: A Cookbook for Elementary Teachers. Dubuque, Iowa: Kendall Hunt. p. 26. ISBN 0-7872-2741-2.
- ^ Cassassa, E. Z.; A. M. Sarquis; C. H. Van Dyke (January 1986). "The Gelation of Polyvinyl Alcohol with Borax". Journal of Chemical Education. 63 (1): 57. doi:10.1021/ed063p57.
- ^ Katoa, Y.; K. Suwaa; S. Yokoyamab; T. Yabeb; H. Ikutaa; Y. Uchimotoa; M. Wakihara (December 2002). "Thermally stable solid polymer electrolyte containing borate ester groups for lithium secondary battery". Solid State Ionics. 152–153: 155–159. doi:10.1016/s0167-2738(02)00370-3.
- Chemistry experiments
- Non-Newtonian fluids