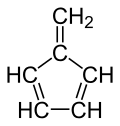
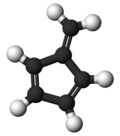
Fulvene
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
5-Methylidenecyclopenta-1,3-diene[1] | |||
| Other names
Fulvene[1]
5-Methylene-1,3-cyclopentadiene | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C6H6 | |||
| Molar mass | 78.114 g·mol−1 | ||
| -42.9·10−6 cm3/mol | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Fulvene (Pentafulvene) usually refers to the hydrocarbon (CH=CH)2C=CH2. It is a prototype of a cross-conjugated hydrocarbon.[2] The parent, fulvene itself, is rarely encountered,[3] but substituted derivatives are numerous. They are mainly of interest as ligands and precursors to ligands in organometallic chemistry. They are often yellow.
Preparation[]
Substituted fulvenes are readily prepared by the condensation of cyclopentadiene and aldehydes and ketones:
- C5H6 + R2C=O → C4H4C=CR2 + H2O
Thiele is credited with discovering this reaction.[4][5]
Modern synthesis of fulvenes employ buffer systems.[6][7]
Fulvenes[]
Several types of fulvenes are defined.[8] They are:
- pentafulvene
- triafulvene
- heptafulvene
- nonafulvene
Ligand in organometallic chemistry[]
Fulvenes are common ligands and ligand precursors in organometallic chemistry.[9] , abbreviated Me4Fv, results from the deprotonation of cationic pentamethylcyclopentadienyl complexes.[10] Some Me4Fv complexes are called tuck-in complexes.

See also[]
References[]
- ^ a b Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 379. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ Preethanuj Preethalayam; Syam krishnan, K.; Sreeja Thulasi; Sarath Chand,S.; Jomy Joseph; Vijay Nair; Florian Jaroschik; K.V.Radhakrishnan (2017). "Recent Advances in the Chemistry of Pentafulvenes". Chemical Reviews. 117 (5): 3930–3989. doi:10.1021/acs.chemrev.6b00210. PMID 28151643.
- ^ Bergmann, E. D. (1968). "Fulvenes and Substituted Fulvenes". Chemical Reviews. 68: 41–84. doi:10.1021/cr60251a002.
- ^ Thiele, J. (1900). "Ueber Ketonreactionen bei dem Cyclopentadiën". Chemische Berichte. 33: 666–673. doi:10.1002/cber.190003301113.
- ^ Hafner, K.; Vöpel, K. H.; Ploss, G.; König, C. (1967). "6-(Dimethylamino)Fulvene". Organic Syntheses. 47: 52. doi:10.15227/orgsyn.047.0052.
- ^ Coşkun, Necdet; Erden, Ihsan (2011-11-11). "An efficient catalytic method for fulvene synthesis". Tetrahedron. 67 (45): 8607–8614. doi:10.1016/j.tet.2011.09.036. ISSN 0040-4020. PMC 3196336. PMID 22021940.
- ^ Sieverding, Paul; Osterbrink, Johanna; Besson, Claire; Kögerler, Paul (2019-01-18). "Kinetics and mechanism of pyrrolidine buffer-catalyzed fulvene formation". J. Org. Chem. 84 (2): 486–494. doi:10.1021/acs.joc.8b01660. ISSN 0022-3263. PMID 30540466.
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "Fulvenes". doi:10.1351/goldbook.F02550
- ^ Strohfeldt, Katja; Tacke, Matthias (2008). "Bioorganometallic fulvene-derived titanocene anti-cancer drugs". Chemical Society Reviews. 37 (6): 1174–87. doi:10.1039/B707310K. PMID 18497930.
- ^ Kreindlin, A. Z.; Rybinskaya, M. A. (2004). "Cationic and Neutral Transition Metal Complexes with a Tetramethylfulvene or Trimethylallyldiene Ligand". Russian Chemical Reviews. 73 (5): 417–432. Bibcode:2004RuCRv..73..417K. doi:10.1070/RC2004v073n05ABEH000842.
- Hydrocarbons
- Vinylidene compounds
- Cyclopentadienes