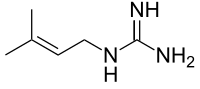
Galegine
| |
| Names | |
|---|---|
| IUPAC name
2-(3-Methylbut-2-enyl)guanidine
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H13N3 | |
| Molar mass | 127.191 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Galegine is a toxic chemical compound that has been isolated from Goat's rue (Galega officinalis).[1] It has also been found to be the principal cause of the toxicity of (Schoenus asperocarpus).[2]
Galegine was used in the 1920s as a pharmaceutical treatment for diabetes;[3] however, because of its toxicity, its use was soon supplanted by superior alternatives. Research based upon the effects of galegine eventually led to the development of metformin which is used today for treatment of type 2 diabetes.[3]
See also[]
References[]
- ^ Oldham, Michelle; Ransom, Corey V.; Ralphs, Michael H.; Gardner, Dale R. (2011). "Galegine Content in Goatsrue (Galega officinalis) Varies by Plant Part and Phenological Growth Stage". Weed Science. 59 (3): 349–352. doi:10.1614/WS-D-10-00169.1.
- ^ Huxtable, C. R.; Dorling, P. R.; Colegate, S. M. (1993). "Identification of galegine, an isoprenyl guanidine, as the toxic principle of Schoenus asperocarpus (poison sedge)". Australian Veterinary Journal. 70 (5): 169–71. doi:10.1111/j.1751-0813.1993.tb06120.x. PMID 8343085.
- ^ a b Bailey, CJ; Day, C. (2004). "Metformin: Its botanical background". Practical Diabetes International. 21 (3): 115–117. doi:10.1002/pdi.606.
Categories:
- Guanidine alkaloids
- Plant toxins