Gentiobiose
| |
| Names | |
|---|---|
| IUPAC name
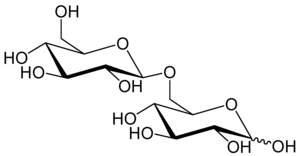
6-O-β-D-glucopyranosyl-D-glucose
| |
| Other names
amygdalose
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C12H22O11 | |
| Molar mass | 342.30 g/mol |
| Density | 1.768 g/mL |
| Melting point | 190 to 195 °C (374 to 383 °F; 463 to 468 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Gentiobiose is a disaccharide composed of two units of D-glucose joined with a β(1->6) linkage. It is a white crystalline solid that is soluble in water or hot methanol. Gentiobiose is incorporated into the chemical structure of crocin, the chemical compound that gives saffron its color. It is a product of the caramelization of glucose.[2]
References[]
- ^ The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals (11th ed.), Merck, 1989, p. 4288, ISBN 091191028X
- ^ Sugisawa, Hirqshi; Edo, Hiroshi (1966). "The Thermal Degradation of Sugars I. Thermal Polymerization of Glucose". Journal of Food Science. 31 (4): 561. doi:10.1111/j.1365-2621.1966.tb01905.x.
Categories:
- Disaccharides
- Organic compound stubs