Hydrogen peroxide - urea
| |||

| |||
| Names | |||
|---|---|---|---|
| Other names
Urea peroxide, percarbamide, UHP
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.004.275 | ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| Properties | |||
| CH6N2O3 | |||
| Molar mass | 94.070 g·mol−1 | ||
| Appearance | White solid | ||
| Density | 1.50 g/cm3 | ||
| Melting point | 75 to 91.5 °C (167.0 to 196.7 °F; 348.1 to 364.6 K) (decomposes) | ||
| Pharmacology | |||
| D02AE01 (WHO) | |||
| Hazards | |||
| Safety data sheet | External MSDS | ||
EU classification (DSD) (outdated)
|
|||
| Flash point | 60 °C (140 °F; 333 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Hydrogen peroxide - urea (also called Hyperol, artizone, urea hydrogen peroxide, and UHP) is a solid composed of equal amounts of hydrogen peroxide and urea. This compound is a white crystalline solid which dissolves in water to give free hydrogen peroxide. Hydrogen peroxide - urea contains solid and water-free hydrogen peroxide, which offers a higher stability and better controllability than liquid hydrogen peroxide when used as an oxidizing agent. Often called carbamide peroxide in the dental office, it is used as a source of hydrogen peroxide for bleaching, disinfection, and oxidation.
Production[]
For the preparation of the complex, urea is dissolved in 30% hydrogen peroxide (molar ratio 2:3) at temperatures below 60 °C. upon cooling this solution, hydrogen peroxide - urea precipitates in the form of small platelets.[1]
Determination of the hydrogen peroxide content by titration with potassium permanganate solution gives a value of 35.4% which corresponds to 97.8% of the theoretical maximum value. The remaining impurity consists of urea.
Akin to water of crystallization, hydrogen peroxide cocrystallizes with urea with the stoichiometry of 1:1. The compound is simply produced (on a scale of several hundred tonnes a year) by the dissolution of urea in excess concentrated hydrogen peroxide solution, followed by crystallization.[2] The laboratory synthesis is analogous.[3]
Structure and properties[]
The solid state structure of this adduct has been determined by neutron diffraction.[4]
Hydrogen peroxide-urea is a readily water-soluble, odorless, crystalline solid, which is available as white powder or colorless needles or platelets.[1] Upon dissolving in various solvents, the 1:1 complex dissociates back to urea and hydrogen peroxide. So just like hydrogen peroxide, the (erroneously) so-called adduct is an oxidizer but the release at room temperature in the presence of catalysts proceeds in a controlled manner, thus the compound is suitable as a safe substitute for the unstable aqueous solution of hydrogen peroxide. Because of the tendency for thermal decomposition, which accelerates at temperatures above 82 °C,[5] it should not be heated above 60 °C, particularly in pure form.
The solubility of commercial samples varies from 0.05 g/mL[6] to more than 0.6 g/mL.[7]
Applications[]
Disinfectant and bleaching agent[]
Hydrogen peroxide - urea is mainly used as a disinfecting and bleaching agent in cosmetics and pharmaceuticals.[2] As a drug, this compound is used in some preparations for the whitening of teeth.[2][8][9] It is also used to relieve minor inflammation of gums, oral mucosal surfaces and lips including canker sores and dental irritation,[10] and to emulsify and disperse earwax.[11]
Carbamide peroxide is also suitable as a disinfectant, e.g. for germ reduction on contact lens surfaces or as an antiseptic for mouthwashes, ear drops or for superficial wounds and ulcers.
Reagent in organic synthesis[]
In the laboratory, it is used as a more easily handled replacement for hydrogen peroxide.[3][12][13] It has proven to be a stable, easy-to-handle and effective oxidizing agent which is readily controllable by a suitable choice of the reaction conditions. It delivers oxidation products in an environmentally friendly manner and often in high yields especially in the presence of organic catalysts such as cis-butenedioic anhydride[14] or inorganic catalysts such as sodium tungstate.[15]

[verification needed]
It converts thiols selectively to disulfides,[14] secondary alcohols to ketones,[15] sulfides to sulfoxides and sulfones,[16] nitriles to amides,[16][17] N-heterocycles to amine oxides.[16][18]

Hydroxybenzaldehyde are converted to dihydroxybenzenes (Dakin reaction)[16][19][better source needed] and gives under suitable conditions the corresponding benzoic acids.[19]
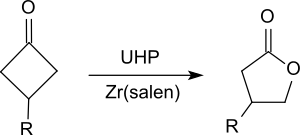
It oxidizes ketones to esters, in particular cyclic ketones, such as substituted cyclohexanones[20] or cyclobutanones[21] to give lactones (Baeyer-Villiger oxidation).
The epoxidation of various alkenes in the presence of benzonitrile yields oxiranes in yields of 79 to 96%.[22]
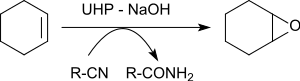
The oxygen atom transferred to the alkene originates from the peroxoimide acid formed intermediately from benzonitrile. The resulting imidic acid tautomerizes to the benzamide.
Safety[]
The compound acts as a strong oxidizing agent and can cause skin irritation and severe eye damage.[23]
See also[]
References[]
- ^ Jump up to: a b C.-S. Lu, E.W. Hughes, P.A. Giguère (1941), "The crystal structure of the urea-hydrogen peroxide addition compound CO(NH2)2 H2O2", J. Am. Chem. Soc., 63 (6), pp. 1507–1513, doi:10.1021/ja01851a007CS1 maint: multiple names: authors list (link)
- ^ Jump up to: a b c Harald Jakob, Stefan Leininger, Thomas Lehmann, Sylvia Jacobi, Sven Gutewort. "Peroxo Compounds, Inorganic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_177.pub2.CS1 maint: multiple names: authors list (link)
- ^ Jump up to: a b Yu, Lei; Meng, Bo; Huang, Xian (2008). "Urea-Hydrogen Peroxide Complex: A Selective Oxidant in the Synthesis of 2-Phenylselenyl-1,3-butadienes". Synthetic Communications. 38 (18): 3142. doi:10.1080/00397910802109224. S2CID 98323467.
- ^ Fritchie, Jr., C. J.; McMullan, R. K. (1981). "Neutron Diffraction Study of the 1:1 Urea:Hydrogen Peroxide complex at 81 K". Acta Crystallographica Section B. 37 (5): 1086. doi:10.1107/S0567740881005116.
- ^ H. Heaney, F. Cardona, A. Goti, A.L. Frederick (2013). "Hydrogen Peroxide-Urea". Encyclopedia of Reagents for Organic Synthesis. E-EROS Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rh047.pub3. ISBN 978-0471936237.CS1 maint: multiple names: authors list (link)
- ^ Sigma-Aldrich specification sheet
- ^ Chemicalland data sheet
- ^ Mokhlis, G. R.; Matis, B. A.; Cochran, M. A.; Eckert, G. J. (2000). "A Clinical Evaluation of Carbamide Peroxide and Hydrogen Peroxide Whitening Agents during Daytime Use". Journal of the American Dental Association. 131 (9): 1269–77. doi:10.14219/jada.archive.2000.0380. PMID 10986827. Archived from the original on 2013-02-23.
- ^ Toothwhitening Archived 2008-03-17 at the Wayback Machine from the UMD of New Jersey website
- ^ Center for Integrative Medicine: Carbamide Peroxide from the University of Maryland Medical Center website Archived October 18, 2007, at the Wayback Machine
- ^ "Ear Drops GENERIC NAME(S): CARBAMIDE PEROXIDE". WebMD. Retrieved July 3, 2021.
- ^ Varma, Rajender S.; Naicker, Kannan P. (1999). "The Urea−Hydrogen Peroxide Complex: Solid-State Oxidative Protocols for Hydroxylated Aldehydes and Ketones (Dakin Reaction), Nitriles, Sulfides, and Nitrogen Heterocycles". Organic Letters. 1 (2): 189. doi:10.1021/ol990522n.
- ^ Harry Heaney, Francesca Cardona, Andrea Goti, "Hydrogen Peroxide–Urea" Encyclopedia of Reagents for Organic Synthesis 2008. doi:10.1002/047084289X.rh047.pub2
- ^ Jump up to: a b B. Karami, M. Montazerozohori, M. H. Habibi (2005), "Urea-Hydrogen Peroxide (UHP) oxidation of thiols to the corresponding disulfides promoted by maleic anhydride as mediator" (PDF), Molecules (in German), 10 (10), pp. 1358–1363, doi:10.3390/10101385, PMC 6147623, PMID 18007530CS1 maint: multiple names: authors list (link)
- ^ Jump up to: a b M. Lukasiewicz; D. Bogdal; J. Pielichowski. "Microwave-assisted oxidation of alcohols using urea hydrogen peroxide". 8th International Electronic Conference on Synthetic Organic Chemistry. ECSOC-8. Retrieved 2016-05-10.
- ^ Jump up to: a b c d R.S. Varma, K.P. Naicker, "The Urea-Hydrogen Peroxide Complex: Solid-State Oxidative Protocols for Hydroxylated Aldehydes and Ketones (Dakin Reaction), Nitriles, Sulfides, and Nitrogen Heterocycles", Org. Lett. (in German), 1 (2), pp. 189–191, doi:10.1021/ol990522n
- ^ US 0
- ^ D. Rong, V.A. Phillips, R.S. Rubio, M.A. Castro, R.T. Wheelhouse, "A safe, convenient and efficient method for the preparation of heterocyclic N-oxides using urea-hydrogen peroxide", Tetrahedron Lett. (in German), 49 (48), pp. 6933–6935, doi:10.1016/j.tetlet.2008.09.124CS1 maint: multiple names: authors list (link)
- ^ Jump up to: a b H. Heaney, A.J. Newbold (2001), "The oxidation of aromatic aldehydes by magnesium monoperoxyphthalate and urea-hydrogen peroxide", Tetrahedron Lett. (in German), 42 (37), pp. 6607–6609, doi:10.1016/S0040-4039(01)01332-6
- ^ M.Y. Rios, E. Salazar, H.F. Olivo (2007), "Baeyer–Villiger oxidation of substituted cyclohexanones via lipase-mediated perhydrolysis utilizing urea–hydrogen peroxide in ethyl acetate", Green Chem. (in German), 9 (5), pp. 459–462, doi:10.1039/B618175ACS1 maint: multiple names: authors list (link)
- ^ A. Watanabe, T. Uchida, K. Ito, T. Katsuki (2002), "Highly enantioselective Baeyer-Villiger oxidation using Zr(salen) complex as catalyst", Tetrahedron Lett. (in German), 43 (25), pp. 4481–4485, doi:10.1016/S0040-4039(02)00831-6CS1 maint: multiple names: authors list (link)
- ^ L. Ji, Y.-N. Wang, C. Qian, X.-Z. Chen (2013), "Nitrile-promoted alkene epoxidation with urea-hydrogen peroxide (UHP)", Synth. Commun. (in German), 43 (16), pp. 2256–2264, doi:10.1080/00397911.2012.699578, S2CID 93770740CS1 maint: multiple names: authors list (link)
- ^
External links[]
- "Hydrogen peroxide urea adduct, UHP". Organic Chemistry Portal.
- "Carbamide Peroxide Monograph". Drugs.com.
- Bleaches
- Antiseptics
- Cleaning product components
- Ureas
- Peroxides
- Oxidizing agents
- Hydrogen peroxide

