MEAI
 | |
| Clinical data | |
|---|---|
| Trade names | Pace |
| Other names | 5-MeO-AI; 2,3-Dihydro-5-methoxy-1H-Inden-2-amine |
| Routes of administration | Oral |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | high |
| Metabolism | acetyl-aminoindandane |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
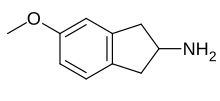
| Formula | C10H13NO |
| Molar mass | 163.220 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
MEAI (5-methoxy-2-aminoindane or 5-MeO-AI or Chaperon) belongs to the indane family of molecules. It was a recreational drug and binge drinking prevention drug. Its molecular structure was first mentioned implicitly in a markush structure schema appearing in a patent from 1998.[1] It was later explicitly and pharmacologically described in a peer reviewed paper in 2017 by David Nutt et al.,[2] followed by another in February 2018 which detailed pharmacokinetics, pharmacodynamics and metabolism of MEAI.[3] One year later it was studied an reported on in another peer reviewed paper.[4] In 2018, a company in the United States began offering an MEAI-based drink called "Pace"[5].The aminoindane family of molecules was, perhaps, first chemically described in 1980.[6][7]
MEAI was an early candidate of alcohol replacement drugs that came to market during a late 2010s movement to replace alcohol with less-toxic alternatives spearheaded by British psychopharmacologist David Nutt.In an act of gonzo journalism, Michael Slezak writing for New Scientist, tried and reported on his experience with MEAI[8]. MEAI was made commercially available online in the United States as "Pace" and is currently being prepared for FDA of registration by Clearmind Medicine Inc (CSE:CMND).[9] The company claims wide Intellectual Property Holding[10][11][12]
See also[]
References[]
- ^ US5708018A, Haadsma-Svensson, Susanne R.; Andersson, Bengt R. & Sonesson, Clas A. et al., "2-aminoindans as selective dopamine D3 ligands", issued 1998-01-13
- ^ Shimshoni JA, Winkler I, Edery N, Golan E, van Wettum R, Nutt D (March 2017). "Toxicological evaluation of 5-methoxy-2-aminoindane (MEAI): Binge mitigating agent in development". Toxicology and Applied Pharmacology. 319: 59–68. doi:10.1016/j.taap.2017.01.018. PMID 28167221. S2CID 205304106.
- ^ Shimshoni JA, Sobol E, Golan E, Ben Ari Y, Gal O (March 2018). "Pharmacokinetic and pharmacodynamic evaluation of 5-methoxy-2-aminoindane (MEAI): A new binge-mitigating agent". Toxicology and Applied Pharmacology. 343: 29–39. doi:10.1016/j.taap.2018.02.009. PMID 29458138. S2CID 3879333.
- ^ Halberstadt, Adam L.; Brandt, Simon D.; Walther, Donna; Baumann, Michael H. (March 2019). "2-Aminoindan and its ring-substituted derivatives interact with plasma membrane monoamine transporters and α2-adrenergic receptors". Psychopharmacology. 236 (3): 989–999. doi:10.1007/s00213-019-05207-1. PMC 6848746.
- ^ "Alcohol Alternative | Redlands | Pacedrink.com". pacedrink. Retrieved 1 March 2022.
- ^ Sainsbury PD, Kicman AT, Archer RP, King LA, Braithwaite RA (2011). "Aminoindanes--the next wave of 'legal highs'?". Drug Testing and Analysis. 3 (7–8): 479–82. doi:10.1002/dta.318. PMID 21748859.
- ^ Cannon JG, Perez JA, Pease JP, Long JP, Flynn JR, Rusterholz DB, Dryer SE (July 1980). "Comparison of biological effects of N-alkylated congeners of beta-phenethylamine derived from 2-aminotetralin, 2-aminoindan, and 6-aminobenzocycloheptene". Journal of Medicinal Chemistry. 23 (7): 745–9. doi:10.1021/jm00181a009. PMID 7190613.
- ^ High and Dry - New Scientist
- ^ "(CSE:CMND)"
- ^ Alcoholic beverage substitutes
- ^ European Patent - Binge Behavior Regulators
- ^ Clearmind Medicine Inc.
- Designer drugs
- 2-Aminoindanes
- Phenol ethers