Magnus's green salt
| |
| |
| Names | |
|---|---|
| IUPAC name
Tetraammineplatinum(II) tetrachloroplatinate(II)
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.034.078 |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| [Pt(NH3)4][PtCl4] | |
| Molar mass | 600.09 g/mol |
| Appearance | green solid |
| Density | 3.7 g/cm3 |
| Melting point | 320 °C (608 °F; 593 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
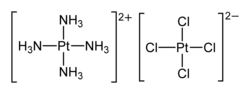
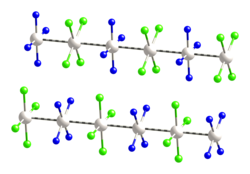
Magnus's green salt is the inorganic compound with the formula [Pt(NH3)4][PtCl4]. This salt is named after Heinrich Gustav Magnus, who, in the early 1830s, first reported the compound. The compound has an unusual structure, consisting of a chain of platinum atoms, and it exhibits unusual properties, being dark green, which is very unusual for platinum compounds.
Structure[]
This species has been of interest in materials chemistry and solid-state physics because of its one-dimensional structure. It contains a linear chain of alternating [PtCl4]2− anions and [Pt(NH3)4]2+ cations, in which the platinum atoms are separated by 3.25 Å.[1] It is a semiconductor.
Preparation[]
The compound may be prepared by combining aqueous solutions of [Pt(NH3)4]2+ and [PtCl4]2−, which gives a deep green solid precipitate.[2] Under some conditions, this reaction affords a pink polymorph of Magnus's green salt. In this so-called "Magnus's pink salt", the square planar Pt complexes are not stacked.[3]
Related compounds[]
Magnus's green salt has the same empirical formula as cis-PtCl2(NH3)2 ("Peyrone chloride") and trans-PtCl2(NH3)2. These cis and trans compounds are molecules, whereas Magnus's green salt is a polymer. This difference is manifested by the solubility of the molecular complexes in water, whereas Magnus's green salt is insoluble.
Soluble analogues of Magnus's green salt can be prepared by replacing the ammonia with ethylhexylamine.[4][5]
The corresponding palladium compound ([Pd(NH3)4PdCl4]) is known as "Vauquelin’s salt".
History[]
Magnus's green salt was one of the first examples of a metal ammine complex. Ammonia species are very common now: they were the basis of Alfred Werner's discoveries.
References[]
- ^ Atoji, M.; Richardson, J. W.; Rundle, R. E. (1957). "On the Crystal Structures of the Magnus Salts, Pt(NH3)4PtCl4". J. Am. Chem. Soc. 79 (12): 3017–3020. doi:10.1021/ja01569a009.
- ^ R. N. Keller (1946). "Tetrammineplatinum(II) Chloride: (Tetrammineplatinous Chloride)". Inorganic Syntheses. Inorganic Syntheses. 2. pp. 250–253. doi:10.1002/9780470132333.ch80. ISBN 9780470132333.
- ^ Lucier, B. E. G. et al. (2014). "Unravelling the Structure of Magnus' Pink Salt" (PDF). Journal of the American Chemical Society. 136 (4): 1333–1351. doi:10.1021/ja4076277. PMID 24437378.CS1 maint: uses authors parameter (link)
- ^ Caseri, W. (2004). "Derivatives of Magnus's green salt; from intractable materials to solution-processed transistors". Platinum Metals Rev. 48 (3): 91–100. doi:10.1595/147106704X1504.
- ^ Bremi, J.; Caseri, W. & Smith, P. (2001). "A new compound derived from Magnus' green salt: solid state structure and evidence for platinum chains in solution". J. Mater. Chem. 11 (10): 2593–2596. doi:10.1039/b104675f.
- Platinum(II) compounds
- Metal halides
- Ammine complexes
- Chloro complexes
- Inorganic polymers