Malaprade reaction
In organic chemistry, the Malaprade reaction or Malaprade oxidation is a glycol cleavage reaction in which a vicinal diol is oxidized by periodic acid or a periodate salt to give the corresponding carbonyl functional groups.[1] The reaction was first reported by Léon Malaprade[2] in 1934[disputed ] and also works with beta-aminoalcohols.[3]
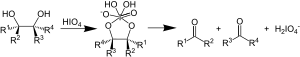
Malaprade reaction

Malaprade reaction mechanism
References[]
- ^ "406. Malaprade Reaction (Malaprade Oxidation)". Comprehensive Organic Name Reactions and Reagents. Wiley. 2010. pp. 1807–1810. doi:10.1002/9780470638859.conrr406.
- ^ L. Malaprade, Bull. Soc. Chim. Fr. 3, 1, 833 1934;
- ^ Nicolet, Ben H.; Shinn, Leo A. (1939). "The Action of Periodic Acid on α-Amino Alcohols". J. Am. Chem. Soc. 61 (6): 1615. doi:10.1021/ja01875a521.
See also[]
Categories:
- Organic chemistry stubs
- Organic oxidation reactions
- Name reactions

