Mallotophenone
| |
| Names | |
|---|---|
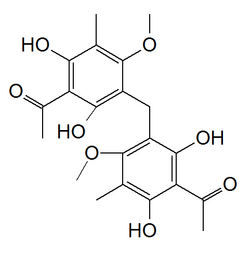
| Preferred IUPAC name
1,1′-[Methylenebis(2,6-dihydroxy-4-methoxy-5-methyl-3,1-phenylene)]di(ethan-1-one) | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C21H24O8 | |
| Molar mass | 404.41 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Mallotophenone is a dimeric phloroglucinol found in Mallotus oppositifolius or in Mallotus japonicus.
The bioassay-guided fractionation of an ethanol extract of the leaves and inflorescence of M. oppositifolius collected in Madagascar led to the isolation of the two new bioactive dimeric phloroglucinols mallotophenone, together with mallotojaponins B and C. These compounds show antiproliferative and antiplasmodial activities.[1]
References[]
- ^ Harinantenaina, Liva; Bowman, Jessica D.; Brodie, Peggy J.; Slebodnick, Carla; Callmander, Martin W.; Rakotobe, Etienne; Randrianaivo, Richard; Rasamison, Vincent E.; Gorka, Alexander; Roepe, Paul D.; Cassera, Maria B.; Kingston, David G. I. (2013). "Antiproliferative and Antiplasmodial Dimeric Phloroglucinols from Mallotus oppositifolius from the Madagascar Dry Forest(1)". Journal of Natural Products. 76 (3): 388–93. doi:10.1021/np300750q. PMC 3606680. PMID 23286240.
Categories:
- Phloroglucinols
- Natural phenol dimers
- Aromatic compound stubs