From Wikipedia, the free encyclopedia
Meleagrin
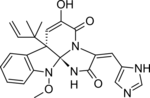
|
| Identifiers
|
|
|
- 71751-77-4
 N N
|
3D model (JSmol)
|
|
| ChEBI
|
|
| ChemSpider
|
|
|
|
|
|
|
|
InChI=1S/C23H23N5O4/c1-5-21(2,3)22-11-18(29)20(31)27-17(10-14-12-24-13-25-14)19(30)26-23(22,27)28(32-4)16-9-7-6-8-15(16)22/h5-13,29H,1H2,2-4H3,(H,24,25)(H,26,30)/b17-10+/t22-,23-/m0/s1 Key: JTJJJLSLKZFEPJ-ZAYCRUKZSA-N InChI=1/C23H23N5O4/c1-5-21(2,3)22-11-18(29)20(31)27-17(10-14-12-24-13-25-14)19(30)26-23(22,27)28(32-4)16-9-7-6-8-15(16)22/h5-13,29H,1H2,2-4H3,(H,24,25)(H,26,30)/b17-10+/t22-,23-/m0/s1 Key: JTJJJLSLKZFEPJ-ZAYCRUKZBE
|
CC(C)(C=C)[C@@]12C=C(C(=O)N\3[C@]1(NC(=O)/C3=C\c4c[nH]cn4)N(c5c2cccc5)OC)O
|
| Properties
|
|
|
C23H23N5O4
|
| Molar mass
|
433.468 g·mol−1
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). |
| Infobox references
|
|
|
|
Chemical compound
Meleagrin and its derivatives such as oxaline are bio-active benzylisoquinoline alkaloids made by deep ocean Penicillium.
Notes[]
Alkaloids from a deep ocean sediment-derived fungus Penicillium sp. and their antitumor activities
Categories:
- Imidazoles
- Indole alkaloids
- Penicillium
- Heterocyclic compounds with 4 rings
- Nitrogen heterocycles
- Alkaloid stubs
Hidden categories:
- Articles without KEGG source
- Articles without UNII source
- Chembox CAS registry number not linked
- Articles with changed CASNo identifier
- Pages using collapsible list with both background and text-align in titlestyle
- Articles containing unverified chemical infoboxes
- Chembox image size set
- Articles with short description
- Short description matches Wikidata
- All stub articles
