Menthofuran
| |
| Names | |
|---|---|
| IUPAC name
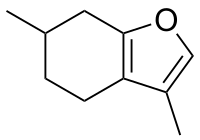
3,6-Dimethyl-4,5,6,7-tetrahydro-1-benzofuran
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.007.087 |
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H14O | |
| Molar mass | 150.221 g·mol−1 |
| Boiling point | 208 |
| Hazards | |
| Flash point | 86 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Menthofuran is an organic compound found in a variety of essential oils including that of pennyroyal (Mentha pulegium). It is highly toxic and believed to be the primary toxin in pennyroyal responsible for its potentially fatal effects.[1] After ingestion of menthofuran, it is metabolically activated to chemically reactive intermediates that are hepatotoxic.[2]
Biosynthesis[]
Menthofuran is produced biosynthetically from pulegone by the enzyme menthofuran synthase.
- Menthofuran synthase converts pulegone to menthofuran
Chemistry[]
Synthesis[]
Menthofuran can be synthesized from 5-methylcyclohexane-1,3-dione and allenyldimethylsulfonium bromide in two steps via a furannulation strategy consisting of enolate addition and rearrangement.[3]
Pharmacology[]
Menthofuran is a metabolite of pulegone. Both in vitro and in vivo studies have found the pulegone metabolite menthofuran to be an inhibitor of CYP2A6.[4][5][6][7]
Menthofuran may deplete glutathione levels, leaving hepatocytes vulnerable to free radical damage.[5]
References[]
- ^ Anderson IB, Mullen WH, Meeker JE, Khojasteh-BakhtSC, Oishi S, Nelson SD, Blanc PD (April 1996). "Pennyroyal toxicity: measurement of toxic metabolite levels in two cases and review of the literature". Annals of Internal Medicine. 124 (8): 726–34. doi:10.7326/0003-4819-124-8-199604150-00004. PMID 8633832.
- ^ Thomassen D, Knebel N, Slattery JT, McClanahan RH, Nelson SD (1992). "Reactive intermediates in the oxidation of menthofuran by cytochromes P-450". Chemical Research in Toxicology. 5 (1): 123–30. doi:10.1021/tx00025a021. PMID 1581528.
- ^ Mariko Aso; Sakamoto, Mizue; Urakawa, Narumi; Kanematsu, Ken (1990). "Furannulation strategy. An efficient synthesis of fused 3-methylfurans". Heterocycles. 31 (6): 1003–6. doi:10.3987/com-90-5392.
- ^ Khojasteh-Bakh, S. C.; Koenigs, L. L.; Peter, R. M.; Trager, W. F.; Nelson, S. D. (July 1998). "(R)-(+)-Menthofuran is a potent, mechanism-based inactivator of human liver cytochrome P450 2A6". Drug Metabolism and Disposition. 26 (7): 701–704. PMID 9660853.
- ^ a b Gordon, W. P.; Huitric, A. C.; Seth, C. L.; McClanahan, R. H.; Nelson, S. D. (February 26, 1989). "The metabolism of the abortifacient terpene, (R)-(+)-pulegone, to a proximate toxin, menthofuran". Drug Metabolism and Disposition. 15 (5): 589–594. PMID 2891472.
- ^ Thomassen, D.; Pearson, P. G.; Slattery, J. T.; Nelson, S. D. (January 17, 1991). "Partial characterization of biliary metabolites of pulegone by tandem mass spectrometry. Detection of glucuronide, glutathione, and glutathionyl glucuronide conjugates". Drug Metabolism and Disposition. 19 (5): 997–104. PMID 1686249.
- ^ Kramlinger VM, von Weymarn LB, Murphy SE (May 2012). "Inhibition and inactivation of cytochrome P450 2A6 and cytochrome P450 2A13 by menthofuran, β-nicotyrine and menthol". Chemico-Biological Interactions. 197 (2–3): 87–92. doi:10.1016/j.cbi.2012.03.009. PMC 3362486. PMID 22486895.
- Monoterpenes
- Furans
- Toxins
- Plant toxins
