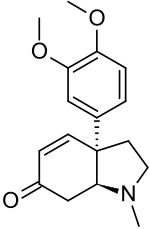
Mesembrenone
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
(3aR,7aS)-3a-(3,4-Dimethoxyphenyl)-1-methyl-1,2,3,3a,7,7a-hexahydro-6H-indol-6-one | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C17H21NO3 | |
| Molar mass | 287.359 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Mesembrenone is an alkaloid constituent of Sceletium tortuosum (Kanna). Similar to modern synthetic antidepressants, it is a potent (IC50 < 1 μM) selective inhibitor of the serotonin transporter (SERT) (that is, a selective serotonin reuptake inhibitor; Ki = 27 nM) and also a phosphodiesterase 4 (PDE4) inhibitor (Ki = 470 nM).[1]
See also[]
- Mesembrine another alkaloid constituent of Kanna.
References[]
- ^ Harvey, A. L.; Young, L. C.; Viljoen, A. M.; Gericke, N. P. (October 2011). "Pharmacological actions of the South African medicinal and functional food plant Sceletium tortuosum and its principal alkaloids". Journal of Ethnopharmacology. 137 (3): 1124–1129. doi:10.1016/j.jep.2011.07.035. PMID 21798331.
Categories:
- Serotonin
- PDE4 inhibitors
- Alkaloids