Methylenation
Methylenation is a chemical reaction that installs the methylene (CH2) group. Typically, the reaction is used to prepare terminal alkenes from aldehydes and, less frequently, ketones.
Methods[]
Methylene-for-oxo reactions[]
A common method for methylenation involves the Wittig reaction using methylenetriphenylphosphorane with an aldehyde (Ph = phenyl or C6H5):[1]
- RCHO + Ph3P=CH2 → RCH=CH2 + Ph3PO
A related reaction can be accomplished with Tebbe's reagent, which is sufficiently versatile to allow methylenation of esters:[2]
- RCO2R' + Cp2Ti(Cl)CH2AlMe2 → RC(OR')=CH2 + "Cp2TiOAlMe2Cl"
Other less well-defined titanium reagents, e.g., Lombardo's reagent, effect similar transformations.[3][4]
Carbanions derived from methylsulfones have also been employed, equivalently to the Wittig reaction.[5]
Other approaches[]
Ethenolysis is a method for methylenation of internal alkene as illustrated by the following example:
- RCH=CHR + CH2=CH2 → 2 RCH=CH2
In principle, the addition of CH2 across a C=C double bond could be classified as a methylenation, but such transformations are commonly described as cyclopropanations.
References[]
- ^ Eric J. Leopold (1986). "Selective Hydroboration of a 1,3,7-Triene: Homogeraniol". Organic Syntheses. 64: 164. doi:10.15227/orgsyn.064.0164.
- ^ Straus, Daniel A.; Morshed, M. Monzur; Dudley, Matthew E.; Hossain, M. Mahmun (2006). "μ-Chlorobis(cyclopentadienyl)(dimethylaluminium)-μ-methylenetitanium". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rc073.pub2. ISBN 0471936235.
- ^ Luciano Lombardo (1987). "Methylenation of Carbonyl Compounds: (+)-3-Methylene-cis-p-menthane". Organic Syntheses. 65: 81. doi:10.15227/orgsyn.065.0081.
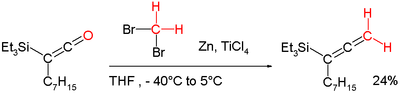
- ^ Marsden, Stephen P; Ducept, Pascal C (2005). "Synthesis of highly substituted allenylsilanes by alkylidenation of silylketenes". Beilstein Journal of Organic Chemistry. 1 (1): 5. doi:10.1186/1860-5397-1-5. PMC 1399453. PMID 16542018.
- ^ Ando, Kaori; Oguchi, Mai; Kobayashi, Takahisa; Asano, Haruka; Uchida, Nariaki (2020). "Methylenation for Aldehydes and Ketones Using 1-Methylbenzimidazol-2-yl Methyl Sulfone". The Journal of Organic Chemistry. 85 (15): 9936–9943. doi:10.1021/acs.joc.0c01227. PMID 32608238.
- Carbon-carbon bond forming reactions