Methylmagnesium chloride
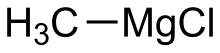
| |
| Names | |
|---|---|
| IUPAC name
chlorido(methyl)magnesium
| |
| Other names
(chloromagnesio)methane
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.010.573 |
| EC Number |
|
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| CH3MgCl | |
| Molar mass | 74.79 g/mol |
| Appearance | colorless solid |
| Reacts with water | |
| Solubility | soluble in diethyl ether and THF |
| Hazards | |
| Main hazards | Flammable, Reacts with water |
| GHS labelling: | |
 
| |
Signal word
|
Danger |
| H225, H250, H260, H314 | |
| P210, P222, P223, P231+P232, P233, P240, P241, P242, P243, P260, P264, P280, P301+P330+P331, P302+P334, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P335+P334, P363, P370+P378, P402+P404, P403+P235, P405, P422, P501 | |
| NFPA 704 (fire diamond) | 
3
3
2 |
| Flash point | −17 °C (1 °F; 256 K) |
| Related compounds | |
Related compounds
|
Phenylmagnesium bromide, Dibutylmagnesium |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Methylmagnesium chloride is an organometallic compound with the general formula CH3MgCl. This highly flammable, colorless, and moisture sensitive material is the simplest Grignard reagent and is commercially available, usually as a solution in tetrahydrofuran.
Synthesis and reactions[]
Relative to the more commonly encountered methylmagnesium bromide[1] and methylmagnesium iodide, methylmagnesium chloride offers the advantages of low equivalent weight and low cost. It is prepared by the reaction of methyl chloride and magnesium in ethyl ether.[2]

As with most Grignard reagents, methylmagnesium chloride is highly solvated by ether solvents via coordination from two oxygen atoms to give a tetrahedrally bonded magnesium center.
Like methyllithium, it is the synthetic equivalent to the methyl carbanion synthon. It reacts with water and other protic reagents to give methane, e.g.,:
- CH3MgCl + ROH → CH4 + MgCl(OR)
When treated with dioxane, methylmagnesium chloride converts to dimethylmagnesium via the Schlenk equilibrium:
- 2 CH3MgCl + dioxane → (CH3)2Mg + MgCl2(dioxane)
See also[]
Further reading[]
- Sakai, Shogo; Jordan, K. D. (1982). "Ab initio study of the structure and vibrational frequencies of the Grignard reagent methylmagnesium chloride". Journal of the American Chemical Society. 104 (14): 4019. doi:10.1021/ja00378a047.
References[]
- ^ Raymond Paul, Olivier Riobé, Michel Maumy (1976). "(E)-4-Hexen-1-ol". Org. Synth. 55: 62. doi:10.15227/orgsyn.055.0062.CS1 maint: uses authors parameter (link)
- ^ E. R. Coburn (1947). "3-Penten-2-ol". Org. Synth. 27: 65. doi:10.15227/orgsyn.027.0065.
- Organomagnesium compounds
- Methylating agents
- Methyl complexes