Nasal vaccine
| Nasal vaccine | |
|---|---|
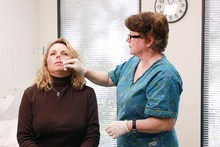
 GI receives an intranasal mist of the flu vaccine at Guantanamo | |
| Specialty | Vaccine delivery |
A nasal vaccine is a vaccine administered to a person via the nose and does not require a needle. It induces immunity through the inner surface of the nose, a surface that naturally comes in contact with many airborne microbes.[1]
Administration[]
Administering a vaccine via the nose is painless, non-invasive and easier to perform than using a needle, which has risks of needle stick injuries and issues relating to safe disposal.[2]
Nasal vaccine technology[]
A few different devices have become available for the nasal delivery of vaccines.[3][4]
Live attenuated influenza vaccine[]

Nasal live attenuated influenza vaccine (LAIV) is available under the brand name FluMist Quadrivalent in the United States and the brand name Fluenz Tetra in Europe.[5][6] In addition to the antigens (active ingredient), nasal flu vaccine contains gelatin,[7] small amounts of amino acids and sucrose, which act as stabilizers.[5][8]
Veterinary medicine[]
In veterinary medicine, vaccination against Bordetella bronchiseptica, a cause of kennel cough, can be delivered by the nasal route in dogs.[9]
History[]
Kangxi Emperor, in the 17th century, claimed that to protect his entire family and army from smallpox he had them all inoculated, a procedure described in manuals of the time as involving the technique of "blowing smallpox material up the nose" or insufflation. The material used varied from ground-up dry scabs to fluid collected from a pustule.[10]
Research[]
Following the September 11 attacks of 2001, and the subsequent anthrax attacks, there has been an active search for vaccines that could be stored and stockpiled, including developments in a nasal vaccine for anthrax.[3]
In an experiment in 2004, a nasal vaccine was given to four African green monkeys in the search for a vaccine for SARS-CoV.[11][12]
In August 2020, during the COVID-19 pandemic, studies in mice and monkeys demonstrated that protection from the new coronavirus might be obtained through the nasal route. Another study postulated that if a COVID-19 vaccine could be given by a spray in the nose, people might be able to vaccinate themselves.[13]
See also[]
References[]
- ^ Scherließ, Regina (2014). "15. Nasal administration of vaccines". In Foged, Camilla; Rades, Thomas; Perrie, Yvonne; Hook, Sarah (eds.). Subunit Vaccine Delivery. Springer. p. 287-306. ISBN 978-1-4939-1417-3.
- ^ Yusuf, Helmy; Kett, Vicky (9 December 2016). "Current prospects and future challenges for nasal vaccine delivery". Human Vaccines & Immunotherapeutics. 13 (1): 34–45. doi:10.1080/21645515.2016.1239668. ISSN 2164-5515. PMC 5287317. PMID 27936348.
- ^ a b Hickey, Anthon J.; Staats, Herman; Roy, Chad J.; Powell, Kenneth; Sullivan, Vince; Rothrock, Ginger; Sayes, Christie M. (2013). "44. Nasal dry powder vaccine delivery technology". In Matthias Giese (ed.). Molecular Vaccines: From Prophylaxis to Therapy. Vol. 2. Springer. p. 719. ISBN 978-3-319-00977-3.
- ^ Lobaina Mato, Yadira (15 December 2019). "Nasal route for vaccine and drug delivery: Features and current opportunities". International Journal of Pharmaceutics. 572: 118813. doi:10.1016/j.ijpharm.2019.118813. ISSN 1873-3476. PMID 31678521.
- ^ a b "Live Attenuated Influenza Vaccine [LAIV] (The Nasal Spray Flu Vaccine) | CDC". www.cdc.gov. 2 September 2020. Retrieved 20 September 2020.
- ^ "Fluenz Tetra nasal spray suspension Influenza vaccine (live attenuated, nasal) - Summary of Product Characteristics (SmPC) - (emc)". www.medicines.org.uk. Retrieved 20 September 2020.
- ^ "Gelatine in vaccines | Vaccine Knowledge". vk.ovg.ox.ac.uk. Retrieved 29 September 2020.
- ^ "Nasal Flu Vaccine : Vaccine Knowledge". vk.ovg.ox.ac.uk. Retrieved 27 September 2020.
- ^ Ford, Richard B. (2009). "197. Companion animal vaccines and vaccination". In Stephen J. Ettinger (ed.). Textbook of Veterinary Internal Medicine. Vol. 1. Edward C. Feldman (7 ed.). Saunders Elsevier. p. 857. ISBN 978-1-4160-6593-7.
- ^ Boylston, Arthur (July 2012). "The origins of inoculation". Journal of the Royal Society of Medicine. 105 (7): 309–313. doi:10.1258/jrsm.2012.12k044. ISSN 0141-0768. PMC 3407399. PMID 22843649.
- ^ Tong, Tomy R. (2006). "Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV)". In Edward Tabor (ed.). Emerging Viruses in Human Populations. Amsterdam: Elsevier. p. 68. ISBN 978-0-444-52074-6.
- ^ Nelson, Laura (2004-06-28). "Nasal solution joins SARS race". Nature. doi:10.1038/news040621-10. ISSN 0028-0836. PMC 7094978.
- ^ "COVID research updates: Immune responses to coronavirus persist beyond 6 months". Nature. 20 November 2020. doi:10.1038/d41586-020-00502-w. PMID 32221507.
- Vaccination
- Drug delivery devices