Nimbin (chemical)
| |
| Names | |
|---|---|
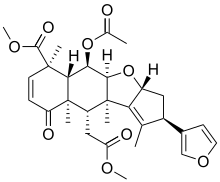
| Preferred IUPAC name
Methyl (2R,3aR,4aS,5R,5aR,6R,9aR,10S,10aR)-5-(acetyloxy)-2-(furan-3-yl)-10-(2-methoxy-2-oxoethyl)-1,6,9a,10a-tetramethyl-9-oxo-3,3a,4a,5,5a,6,9,9a,10,10a-decahydro-2H-cyclopenta[b]naphtho[2,3-d]furan-6-carboxylate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.106.899 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C30H36O9 | |
| Molar mass | 540.609 g·mol−1 |
| Melting point | 205 °C (401 °F; 478 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Nimbin is a triterpenoid isolated from Neem. Nimbin is thought to be responsible for much of the biological activities of neem oil, and is reported to have anti-inflammatory, antipyretic, fungicidal, antihistamine and antiseptic properties.[2]
See also[]
- Azadirachtin, another chemical isolated from neem that is used commercially as an insecticide
References[]
- ^ Siddiqui, Salimuzzaman (1945). "Utilization of nim oil and its bitter constituents (nimbidin series) in the pharmaceutical industry". Journal of Scientific & Industrial Research. 4: 5–10.
- ^ W. Kraus, "Biologically active ingredients-azadirachtin and other triterpenoids", in: H. Schutterer (Ed.), The Neem Tree Azadirachta indica A. Juss and Other Meliaceous Plants, Weinheim, New York, 1995, p 35-88
Categories:
- Triterpenes
- Furans
- Acetate esters
- Methyl esters
- Cyclopentenes
- Ester stubs