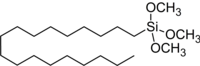
Octadecyltrimethoxysilane
| |
| Names | |
|---|---|
| Preferred IUPAC name
Trimethoxy(octadecyl)silane | |
| Other names
n-Octadecyltrimethoxysilane
Trimethoxyoctadecylsilane | |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | OTMS |
| 5791830 | |
| ChemSpider | |
| ECHA InfoCard | 100.019.400 |
| EC Number |
|
| MeSH | n-Octadecyltrimethoxysilane |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C21H46O3Si | |
| Molar mass | 374.681 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 0.883 g cm−3 |
| Melting point | 16 to 17 °C (61 to 63 °F; 289 to 290 K) |
| Boiling point | 170 °C (338 °F; 443 K) |
Refractive index (nD)
|
1.438-1.44 |
| Hazards[1] | |
| Safety data sheet (SDS) | [1] |
| GHS labelling: | |

| |
Signal word
|
Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | 
2
1
0 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Octadecyltrimethoxysilane (OTMS) is an organosilicon compound. This colorless liquid is used for preparing hydrophobic coatings and self-assembled monolayers. It is sensitive toward water, irreversibly degrading to a siloxane polymer.[2] It places a C18H39SiO3 "cap" on oxide surfaces. The formation of OTMS monolayers is used for converting hydrophilic surfaces to hydrophobic surfaces, e.g. for use in certain areas of nanotechnology and analytical chemistry.
References[]
- ^ "Octadecyltrimethoxysilane". pubchem.ncbi.nlm.nih.gov. Retrieved 5 December 2021.
- ^ P. Fontaine; F. Rondelez (1995). J. Daillant; P. Guenoun; C. Marques; P. Muller; J. Tran Thanh Van (eds.). Kinetics of Polymerisation in Langmuir Monolayers of n-Alkyltrimethoxysilane. Short and Long Chains at Interfaces.
Further reading[]
- Hild, R; David, C; Müller, H. U; Völkel, B; Kayser, D. R; Grunze, M (1998). "Formation and Characterization of Self-assembled Monolayers of Octadecyltrimethoxysilane on Chromium: Application in Low-Energy Electron Lithography". Langmuir. 14 (2): 342–346. doi:10.1021/la970438l.
- Vidon, S; Leblanc, R. M (1998). "Langmuir Study of Octadecyltrimethoxysilane Behavior at the Air−Water Interface". The Journal of Physical Chemistry B. 102 (7): 1279–1286. doi:10.1021/jp973334s.
Categories:
- Silanes
- Methoxy compounds