Optogenetics
Optogenetics is a biological technique that involves the use of light to control neurons that have been genetically modified to express light-sensitive ion channels. As such, optogenetics is a neuromodulation method that uses a combination of techniques from optics and genetics to control the activities of individual neurons in living tissue — even within freely-moving animals.[1] In some usages, optogenetics also refers to optical monitoring of neuronal activity[1] or control of other biochemical pathways in non-neuronal cells (see "Cellular biology/cell signaling pathways" section below),[2] although these research activities preceded the use of light-sensitive ion channels in neurons.[3][4] As optogenetics is used by some authors to refer to only optical control of the activity of genetically defined neurons and not these additional research approaches,[5][6][7] the term optogenetics is an example of polysemy.
Neuronal control is achieved using optogenetic actuators like channelrhodopsin, halorhodopsin, and archaerhodopsin, while optical recording of neuronal activities can be made with the help of optogenetic sensors for calcium (GCaMPs), vesicular release (synapto-pHluorin), neurotransmitters (GluSnFRs), or membrane voltage (Quasars, Accelerated Sensor of Action Potentials, Archons).[8][9] Control (or recording) of activity is restricted to genetically defined neurons and performed in a spatiotemporal-specific manner by light.
In 2010, optogenetics was chosen as the "Method of the Year" across all fields of science and engineering by the interdisciplinary research journal Nature Methods.[10] At the same time, optogenetics was highlighted in the article on "Breakthroughs of the Decade" in the academic research journal Science.[11][12][7]
History[]
In 1979, Francis Crick suggested that controlling all cells from one type in the brain while leaving the others more or less unaltered is a real challenge for neuroscience. Francis Crick speculated that a technology using light might be useful to control neuronal activity with temporal and spatial precision but at the time there was no technique to make neurons responsive to light.
By early 1990s LC Katz and E Callaway had shown that light could uncage glutamate.[13] Heberle and Büldt in 1994 had already shown functional heterologous expression of a bacteriorhodopsin for light-activated ion flow in yeast.[14] Later in 1995, Georg Nagel et al. and Ernst Bamberg tried the heterologous expression of microbial rhodopsins (also bacteriorhodopsin and also in a non-neural system, Xenopus oocytes) (Nagel et al., 1995, FEBS Lett.) and showed light-induced current.
An earlier use of light to activate neurons was carried out by Richard Fork,[15] who demonstrated laser activation of neurons within intact tissue, although not in a genetically-targeted manner. The earliest genetically targeted method that used light to control rhodopsin-sensitized neurons was reported in January 2002, by Boris Zemelman and Gero Miesenböck, who employed Drosophila rhodopsin cultured mammalian neurons.[16] In 2003, Zemelman and Miesenböck developed a second method for light-dependent activation of neurons in which single ionotropic channels TRPV1, TRPM8 and P2X2 were gated by photocaged ligands in response to light.[17] Beginning in 2004, the Kramer and Isacoff groups developed organic photoswitches or "reversibly caged" compounds in collaboration with the Trauner group that could interact with genetically introduced ion channels.[18][19] TRPV1 methodology, albeit without the illumination trigger, was subsequently used by several laboratories to alter feeding, locomotion and behavioral resilience in laboratory animals.[20][21][22] However, light-based approaches for altering neuronal activity were not applied outside the original laboratories, likely because the easier to employ channelrhodopsin was cloned soon thereafter.[23]
Peter Hegemann, studying the light response of green algae at the University of Regensburg, had discovered photocurrents that were too fast to be explained by the classic g-protein-coupled animal rhodopsins.[24] Teaming up with the electrophysiologist Georg Nagel at the Max Planck Institute in Frankfurt, they could demonstrate that a single gene from the alga Chlamydomonas produced large photocurrents when expressed in the oocyte of a frog.[25] To identify expressing cells, they replaced the cytoplasmic tail of the algal protein with the fluorescent protein YFP, generating the first generally applicable optogenetic tool.[23] They stated in the 2003 paper that "expression of ChR2 in oocytes or mammalian cells may be used as a powerful tool to increase cytoplasmic Ca2+ concentration or to depolarize the cell membrane, simply by illumination".
Karl Deisseroth in the Bioengineering Department at Stanford published the notebook pages from early July 2004 of his initial experiment showing light activation of neurons expressing a channelrhodopsin.[26] In August 2005, his laboratory staff, including graduate students Ed Boyden and Feng Zhang, in collaboration with Georg Nagel, published the first demonstration of a single-component optogenetic system, in neurons[27] using the channelrhodopsin-2(H134R)-eYFP construct from Nagel and Hegemann.[23]
Zhuo-Hua Pan of Wayne State University, researching on restore sight to blindness, tried channelrhodopsin out in ganglion cells—the neurons in our eyes that connect directly to the brain. Pan's first observation of optical activation of retinal neurons with channelrhodopsin was in August 2004 according to Pan,[28] a month after Deisseroth's initial observation. Indeed, the transfected neurons became electrically active in response to light, and in 2005, Zhuo-Hua Pan reported successful in-vivo transfection of channelrhodopsin in retinal ganglion cells of mice, and electrical responses to photostimulation in retinal slice culture.[29]
In April 2005, Susana Lima and Miesenböck reported the first use of genetically-targeted P2X2 photostimulation to control the behaviour of an animal.[30] They showed that photostimulation of genetically circumscribed groups of neurons, such as those of the dopaminergic system, elicited characteristic behavioural changes in fruit flies.
In October 2005, Lynn Landmesser and Stefan Herlitze also published the use of channelrohodpsin-2 to control neuronal activity in cultured hippocampal neurons and chicken spinal cord circuits in intact developing embryos.[31] In addition, they introduced for the first time vertebrate rhodopsin, a light-activated G protein coupled receptor, as a tool to inhibit neuronal activity via the recruitment of intracellular signaling pathways also in hippocampal neurons and the intact developing chicken embryo.[31]
The groups of Alexander Gottschalk and Georg Nagel made the first ChR2 mutant (H134R) and were first to use channelrhodopsin-2 for controlling neuronal activity in an intact animal, showing that motor patterns in the roundworm Caenorhabditis elegans could be evoked by light stimulation of genetically selected neural circuits (published in December 2005).[32] In mice, controlled expression of optogenetic tools is often achieved with cell-type-specific Cre/loxP methods developed for neuroscience by Joe Z. Tsien back in the 1990s[33] to activate or inhibit specific brain regions and cell-types in vivo.[34]
In 2007, the labs of Edward Boyden and Karl Deisseroth (together with the groups of Alexander Gottschalk and Georg Nagel) simultaneously reported successful optogenetic inhibition of activity in neurons.[35][36]
In 2007, Georg Nagel group and Peter Hegemann group started the optogenetic manipulation of cAMP.[37] In 2014, Avelar et al. reported the first rhodopsin-guanylyl cyclase gene from fungus. In 2015, Scheib et al. and Gao et al. characterized the activity of the rhodopsin-guanylyl cyclase gene. And Shiqiang Gao et al. and Georg Nagel, Alexander Gottschalk identified it as the first 8 TM enzyme rhodopsin.[38]
Prior to the development of optogentic actuators, optogenetic sensors of activity were developed, for example genetically encoded calcium indicators (GECIs). The first GECI to be used to image activity in an animal was cameleon, designed by Atsushi Miyawaki, Roger Tsien and coworkers in 1997.[4] Cameleon was first used successfully in an animal by Rex Kerr, William Schafer and coworkers to record from neurons and muscle cells of the nematode C. elegans.[39] Cameleon was subsequently used to record neural activity in flies[40] and zebrafish.[41] In mammals, the first GECI to be used in vivo was GCaMP,[42] first developed by Nakai and coworkers.[43] GCaMP has undergone numerous improvements, and GCaMP6[44] in particular has become widely used throughout neuroscience.
Awards[]
The powerful impact of optogenetic technology on brain research has been recognized by numerous awards to key players in the field.
In 2010, Georg Nagel, Peter Hegemann and Ernst Bamberg were awarded the Wiley Prize in Biomedical Sciences.[45] Georg Nagel, Peter Hegemann and Ernst Bamberg were also awarded the Karl Heinz Beckurts Prize in 2010.[46] In 2010, Deisseroth was awarded the inaugural HFSP Nakasone Award "for his pioneering work on the development of optogenetic methods for studying the function of neuronal networks underlying behavior".[47]
In 2012 Georg Nagel, Peter Hegemann, Ernst Bamberg and Deisseroth were awarded the Zülch Prize. In 2012, Miesenböck was awarded the Baillet Latour Health Prize for "pioneering optogenetic approaches to manipulate neuronal activity and to control animal behaviour."[48]
In 2013 Nagel and Peter Hegemann were awarded the Louis-Jeantet Prize for Medicine,.[49] In 2013, Bamberg, Boyden, Deisseroth, Hegemann, Miesenböck and Nagel were awarded The Brain Prize for "their invention and refinement of optogenetics."[50][51]
In 2017, Deisseroth was awarded the Else Kröner Fresenius Research Prize 2017 for "his discoveries in optogenetics and hydrogel-tissue chemistry". Deisseroth was named the laureate of the 2018 Kyoto Prize "for the development of optogenetics and causal systems neuroscience"[52] and the 2020 Heineken Prize in Medicine from the Royal Netherlands Academy of Arts and Sciences, for developing optogenetics.[53]
In 2019, Ernst Bamberg, Georg Nagel, Ed Boyden, Karl Deisseroth, Peter Hegemann and Gero Miesenböck were awarded the Rumford Prize for "extraordinary contributions related to the invention and refinement of optogenetics."[54] In 2020, Miesenböck, Hegemann and Georg Nagel jointly received the Shaw prize in Life Science and Medicine for "the development of optogenetics".
Description[]
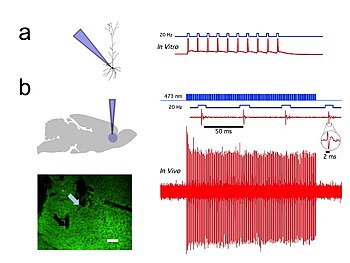
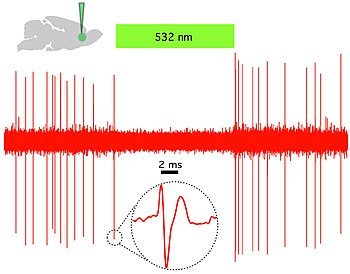
Optogenetics provides millisecond-scale temporal precision which allows the experimenter to keep pace with fast biological information processing (for example, in probing the causal role of specific action potential patterns in defined neurons). Indeed, to probe the neural code, optogenetics by definition must operate on the millisecond timescale to allow addition or deletion of precise activity patterns within specific cells in the brains of intact animals, including mammals (see Figure 1). By comparison, the temporal precision of traditional genetic manipulations (employed to probe the causal role of specific genes within cells, via "loss-of-function" or "gain of function" changes in these genes) is rather slow, from hours or days to months. It is important to also have fast readouts in optogenetics that can keep pace with the optical control. This can be done with electrical recordings ("optrodes") or with reporter proteins that are biosensors, where scientists have fused fluorescent proteins to detector proteins. An example of this is voltage-sensitive fluorescent protein (VSFP2).[58] Additionally, beyond its scientific impact optogenetics represents an important case study in the value of both ecological conservation (as many of the key tools of optogenetics arise from microbial organisms occupying specialized environmental niches), and in the importance of pure basic science as these opsins were studied over decades for their own sake by biophysicists and microbiologists, without involving consideration of their potential value in delivering insights into neuroscience and neuropsychiatric disease.[59]
Light-activated proteins: channels, pumps and enzymes
The hallmark of optogenetics therefore is introduction of fast light-activated channels, pumps, and enzymes that allow temporally precise manipulation of electrical and biochemical events while maintaining cell-type resolution through the use of specific targeting mechanisms. Among the microbial opsins which can be used to investigate the function of neural systems are the channelrhodopsins (ChR2, ChR1, VChR1, and SFOs) to excite neurons and anion-conducting channelrhodopsins for light-induced inhibition. Indirectly light-controlled potassium channels have recently been engineered to prevent action potential generation in neurons during blue light illumination.[60][61] Light-driven ion pumps are also used to inhibit neuronal activity, e.g. halorhodopsin (NpHR),[62] enhanced halorhodopsins (eNpHR2.0 and eNpHR3.0, see Figure 2),[63] archaerhodopsin (Arch), fungal opsins (Mac) and enhanced bacteriorhodopsin (eBR).[64]
Optogenetic control of well-defined biochemical events within behaving mammals is now also possible. Building on prior work fusing vertebrate opsins to specific G-protein coupled receptors[65] a family of chimeric single-component optogenetic tools was created that allowed researchers to manipulate within behaving mammals the concentration of defined intracellular messengers such as cAMP and IP3 in targeted cells.[66] Other biochemical approaches to optogenetics (crucially, with tools that displayed low activity in the dark) followed soon thereafter, when optical control over small GTPases and adenylyl cyclase was achieved in cultured cells using novel strategies from several different laboratories.[67][68][69] Photoactivated adenylyl cyclases have been discovered in fungi and successfully used to control cAMP levels in mammalian neurons.[70][71] This emerging repertoire of optogenetic actuators now allows cell-type-specific and temporally precise control of multiple axes of cellular function within intact animals.[72]
Hardware for light application
Another necessary factor is hardware (e.g. integrated fiberoptic and solid-state light sources) to allow specific cell types, even deep within the brain, to be controlled in freely behaving animals. Most commonly, the latter is now achieved using the fiberoptic-coupled diode technology introduced in 2007,[73][74][75] though to avoid use of implanted electrodes, researchers have engineered ways to inscribe a "window" made of zirconia that has been modified to be transparent and implanted in mice skulls, to allow optical waves to penetrate more deeply to stimulate or inhibit individual neurons.[76] To stimulate superficial brain areas such as the cerebral cortex, optical fibers or LEDs can be directly mounted to the skull of the animal. More deeply implanted optical fibers have been used to deliver light to deeper brain areas.[77] Complementary to fiber-tethered approaches, completely wireless techniques have been developed utilizing wirelessly delivered power to headborne LEDs for unhindered study of complex behaviors in freely behaving organisms.[78] Recent progress investigate the use of organic LEDs (OLEDs) as stimuli for optogenetics.[79] The precise and controlled stimulation of neurons expressing microbial opsin has been demonstrated in vitro on a time scale in the order of a millisecond. Pulsed mode operation allows neural stimulation within compatible low temperature. Moreover, organic light-emitting diodes (OLED) are suitable for implantation in the brain for their very thin thickness which can be less than 1 µm.[79]
Expression of optogenetic actuators
Optogenetics also necessarily includes the development of genetic targeting strategies such as cell-specific promoters or other customized conditionally-active viruses, to deliver the light-sensitive probes to specific populations of neurons in the brain of living animals (e.g. worms, fruit flies, mice, rats, and monkeys). In invertebrates such as worms and fruit flies some amount of all-trans-retinal (ATR) is supplemented with food. A key advantage of microbial opsins as noted above is that they are fully functional without the addition of exogenous co-factors in vertebrates.[75]
Technique[]
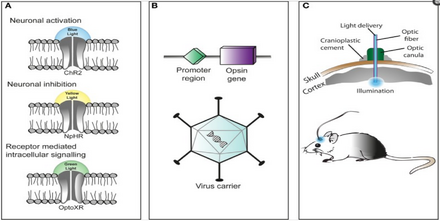
The technique of using optogenetics is flexible and adaptable to the experimenter's needs. For starters, experimenters genetically engineer a microbial opsin based on the gating properties (rate of excitability, refractory period, etc.) required for the experiment.
There is a challenge in introducing the microbial opsin, an optogenetic actuator, into a specific region of the organism in question. A rudimentary approach is to introduce an engineered viral vector that contains the optogenetic actuator gene attached to a recognizable promoter such as CAMKIIα. This allows for some level of specificity as cells that already contain and can translate the given promoter will be infected with the viral vector and hopefully express the optogenetic actuator gene.[81]
Another approach is the creation of transgenic mice where the optogenetic actuator gene is introduced into mice zygotes with a given promoter, most commonly Thy1. Introduction of the optogenetic actuator at an early stage allows for a larger genetic code to be incorporated and as a result, increases the specificity of cells to be infected.
A third and rather novel approach that has been developed is creating transgenic mice with Cre recombinase, an enzyme that catalyzes recombination between two lox-P sites. Then by introducing an engineered viral vector containing the optogenetic actuator gene in between two lox-P sites, only the cells containing the Cre recombinase will express the microbial opsin. This last technique has allowed for multiple modified optogenetic actuators to be used without the need to create a whole line of transgenic animals every time a new microbial opsin is needed.[82]
After the introduction and expression of the microbial opsin, depending on the type of analysis being performed, application of light can be placed at the terminal ends or the main region where the infected cells are situated. Light stimulation can be performed with a vast array of instruments from light-emitting diodes (LEDs) or diode-pumped solid-state laser (DPSS). These light sources are most commonly connected to a computer through a fiber optic cable. Recent advances include the advent of wireless head-mounted devices that also apply LED to targeted areas and as a result give the animal more freedom of mobility to reproduce in vivo results.[83][84]
Moreover, fiber-based approaches can now offer simultaneous single-cellular resolution optical stimulation and Calcium imaging.[77] This enables researchers to visualize and manipulate the activity of single neurons while preserving naturalistic animal behaviours.[85] Further, these techniques allow one to record in multiple deep brain regions at the same using GRIN lenses connected via optical fiber to an externally positioned photodetector and photostimulator.[86][87]
Issues[]
Although already a powerful scientific tool, optogenetics, according to Doug Tischer & Orion D. Weiner of the University of California San Francisco, should be regarded as a "first-generation GFP" because of its immense potential for both utilization and optimization.[88] With that being said, the current approach to optogenetics is limited primarily by its versatility. Even within the field of neuroscience where it is most potent, the technique is less robust on a subcellular level.[89] Further issues are raised by the spatial response at the level of neural networks.
Selective expression[]
One of the main problems of optogenetics is that not all the cells in question may express the microbial opsin gene at the same level. Thus, even illumination with a defined light intensity will have variable effects on individual cells. Optogenetic stimulation of neurons in the brain is even less controlled as the light intensity drops exponentially from the light source (e.g. implanted optical fiber).
Moreover, mathematical modelling shows that selective expression of opsin in specific cell types can dramatically alter the dynamical behavior of the neural circuitry. In particular, optogenetic stimulation that preferentially targets inhibitory cells can transform the excitability of the neural tissue from Type 1 — where neurons operate as integrators — to Type 2 where neurons operate as resonators.[90]
Type 1 excitable media sustain propagating waves of activity whereas Type 2 excitable media do not. The transformation from one to the other explains how constant optical stimulation of primate motor cortex elicits gamma-band (40–80 Hz) oscillations in the manner of a Type 2 excitable medium. Yet those same oscillations propagate far into the surrounding tissue in the manner of a Type 1 excitable medium.[91]
It remains difficult to target opsin to defined subcellular compartments, e.g. the plasma membrane, synaptic vesicles, or mitochondria.[63][89] Restricting the opsin to specific regions of the plasma membrane such as dendrites, somata or axon terminals would provide a more robust understanding of neuronal circuitry.[89]
Kinetics and synchronization[]
An issue with channelrhodopsin-2 is that its gating properties do not mimic in vivo cation channels of cortical neurons. A solution to this issue with a protein's kinetic property is introduction of variants of channelrhodopsin-2 with more favorable kinetics.[55][56]
Another one of the technique's limitations is that light stimulation produces a synchronous activation of infected cells and this removes any individual cell properties of activation among the population affected. Therefore, it is difficult to understand how the cells in the population affected communicate with one another or how their phasic properties of activation may relate to the circuitry being observed.
Optogenetic activation has been combined with functional magnetic resonance imaging (ofMRI) to elucidate the connectome, a thorough map of the brain's neural connections. The results, however, are limited by the general properties of fMRI.[89][92] The readouts from this neuroimaging procedure lack the spatial and temporal resolution appropriate for studying the densely packed and rapid-firing neuronal circuits.[92]
Light absorption spectrum[]
The opsin proteins currently in use have absorption peaks across the visual spectrum, but remain considerably sensitive to blue light.[89] This spectral overlap makes it very difficult to combine opsin activation with genetically encoded indicators (GEVIs, GECIs, GluSnFR, synapto-pHluorin), most of which need blue light excitation. Opsins with infrared activation would, at a standard irradiance value, increase light penetration and augment resolution through reduction of light scattering.
Additional data indicates that the absorption spectra of organic dyes and fluorescent proteins, used in optogenetics applications, extends from around 250 nm to around 600 nm. Particular organic compounds used in discrete portions of this range include: retinals, flavins, folates, p-coumaric acids, phytochrome chromophotes, cobalamins, and at least six fluorescent proteins including mOrange and mCherry.[93]
Spatial response[]
Using a narrow Gaussian light beam to stimulate neurons in a patch of neural tissue can evoke a response profile that is much broader than the stimulation profile.[94] In this case, neurons may be activated (or inhibited) unintentionally. There are computational simulation tools[95][96] which can help to counter this by estimating the impact of optogenetic stimulation before conducting experiments.
Applications[]
The field of optogenetics has furthered the fundamental scientific understanding of how specific cell types contribute to the function of biological tissues such as neural circuits in vivo (see references from the scientific literature below). Moreover, on the clinical side, optogenetics-driven research has led to insights into Parkinson's disease[97][98] and other neurological and psychiatric disorders. Indeed, optogenetics papers in 2009 have also provided insight into neural codes relevant to autism, Schizophrenia, drug abuse, anxiety, and depression.[64][99][100][101] Optogenetics has also been used in an experimental treatment for blindness by which a protein produced due to gene editing is stimulated with light by engineered goggles.[102][103]
Identification of particular neurons and networks[]
Amygdala[]
Optogenetic approaches have been used to map neural circuits in the amygdala that contribute to fear conditioning.[104][105][106][107] One such example of a neural circuit is the connection made from the basolateral amygdala to the dorsal-medial prefrontal cortex where neuronal oscillations of 4 Hz have been observed in correlation to fear induced freezing behaviors in mice. Transgenic mice were introduced with channelrhodoposin-2 attached with a parvalbumin-Cre promoter that selectively infected interneurons located both in the basolateral amygdala and the dorsal-medial prefrontal cortex responsible for the 4 Hz oscillations. The interneurons were optically stimulated generating a freezing behavior and as a result provided evidence that these 4 Hz oscillations may be responsible for the basic fear response produced by the neuronal populations along the dorsal-medial prefrontal cortex and basolateral amygdala.[108]
Olfactory bulb[]
Optogenetic activation of olfactory sensory neurons was critical for demonstrating timing in odor processing[109] and for mechanism of neuromodulatory mediated olfactory guided behaviors (e.g. aggression, mating)[110] In addition, with the aid of optogenetics, evidence has been reproduced to show that the "afterimage" of odors is concentrated more centrally around the olfactory bulb rather than on the periphery where the olfactory receptor neurons would be located. Transgenic mice infected with channel-rhodopsin Thy1-ChR2, were stimulated with a 473 nm laser transcranially positioned over the dorsal section of the olfactory bulb. Longer photostimulation of mitral cells in the olfactory bulb led to observations of longer lasting neuronal activity in the region after the photostimulation had ceased, meaning the olfactory sensory system is able to undergo long term changes and recognize differences between old and new odors.[111]
Nucleus accumbens[]
Optogenetics, freely moving mammalian behavior, in vivo electrophysiology, and slice physiology have been integrated to probe the cholinergic interneurons of the nucleus accumbens by direct excitation or inhibition. Despite representing less than 1% of the total population of accumbal neurons, these cholinergic cells are able to control the activity of the dopaminergic terminals that innervate medium spiny neurons (MSNs) in the nucleus accumbens.[112] These accumbal MSNs are known to be involved in the neural pathway through which cocaine exerts its effects, because decreasing cocaine-induced changes in the activity of these neurons has been shown to inhibit cocaine conditioning. The few cholinergic neurons present in the nucleus accumbens may prove viable targets for pharmacotherapy in the treatment of cocaine dependence[64]
Prefrontal cortex[]

In vivo and in vitro recordings of individual CAMKII AAV-ChR2 expressing pyramidal neurons within the prefrontal cortex demonstrated high fidelity action potential output with short pulses of blue light at 20 Hz (Figure 1).[55]
Motor cortex
In vivo repeated optogenetic stimulation in healthy animals was able to eventually induce seizures.[113] This model has been termed optokindling.
Piriform cortex
In vivo repeated optogenetic stimulation of pyramidal cells of the piriform cortex in healthy animals was able to eventually induce seizures.[114] In vitro studies have revealed a loss of feedback inhibition in the piriform circuit due to impaired GABA synthesis.[114]
Heart[]
Optogenetics was applied on atrial cardiomyocytes to end spiral wave arrhythmias, found to occur in atrial fibrillation, with light.[115] This method is still in the development stage. A recent study explored the possibilities of optogenetics as a method to correct for arrythmias and resynchronize cardiac pacing. The study introduced channelrhodopsin-2 into cardiomyocytes in ventricular areas of hearts of transgenic mice and performed in vitro studies of photostimulation on both open-cavity and closed-cavity mice. Photostimulation led to increased activation of cells and thus increased ventricular contractions resulting in increasing heart rates. In addition, this approach has been applied in cardiac resynchronization therapy (CRT) as a new biological pacemaker as a substitute for electrode based-CRT.[116] Lately, optogenetics has been used in the heart to defibrillate ventricular arrhythmias with local epicardial illumination,[117] a generalized whole heart illumination[118] or with customized stimulation patterns based on arrhythmogenic mechanisms in order to lower defibrillation energy.[119]
Spiral ganglion[]
Optogenetic stimulation of the spiral ganglion in deaf mice restored auditory activity.[120] Optogenetic application onto the cochlear region allows for the stimulation or inhibition of the spiral ganglion cells (SGN). In addition, due to the characteristics of the resting potentials of SGN's, different variants of the protein channelrhodopsin-2 have been employed such as Chronos,[121] CatCh and f-Chrimson.[122] Chronos and CatCh variants are particularly useful in that they have less time spent in their deactivated states, which allow for more activity with less bursts of blue light emitted. Additionally, using engineered red-shifted channels as f-Chrimson allow for stimulation using longer wavelengths, which decreases the potential risks of phototoxicity in the long term without compromising gating speed.[123] The result being that the LED producing the light would require less energy and the idea of cochlear prosthetics in association with photo-stimulation, would be more feasible.[124]
Brainstem[]
Optogenetic stimulation of a modified red-light excitable channelrhodopsin (ReaChR) expressed in the facial motor nucleus enabled minimally invasive activation of motoneurons effective in driving whisker movements in mice.[125] One novel study employed optogenetics on the Dorsal Raphe Nucleus to both activate and inhibit dopaminergic release onto the ventral tegmental area. To produce activation transgenic mice were infected with channelrhodopsin-2 with a TH-Cre promoter and to produce inhibition the hyperpolarizing opsin NpHR was added onto the TH-Cre promoter. Results showed that optically activating dopaminergic neurons led to an increase in social interactions, and their inhibition decreased the need to socialize only after a period of isolation.[126]
Visual system[]
Studying the visual system using optogenetics can be challenging. Indeed, the light used for optogenetic control may lead to the activation of photoreceptors, as a result of the proximity between primary visual circuits and these photoreceptors. In this case, spatial selectivity is difficult to achieve (particularly in the case of the fly optic lobe). Thus, the study of the visual system requires spectral separation, using channels that are activated by different wavelengths of light than rhodopsins within the photoreceptors (peak activation at 480 nm for Rhodopsin 1 in Drosophila). Red-shifted CsChrimson[127] or bistable Channelrhodopsin[128] are used for optogenetic activation of neurons (i.e. depolarization), as both allow spectral separation. In order to achieve neuronal silencing (i.e. hyperpolarization), an anion channelrhodopsin discovered in the cryptophyte algae species Guillardia theta (named GtACR1).[129] can be used. GtACR1 is more light sensitive than other inhibitory channels such as the Halorhodopsin class of chlorid pumps and imparts a strong conductance. As its activation peak (515 nm) is close to that of Rhodopsin 1, it is necessary to carefully calibrate the optogenetic illumination as well as the visual stimulus. The factors to take into account are the wavelength of the optogenetic illumination (possibly higher than the activation peak of GtACR1), the size of the stimulus (in order to avoid the activation of the channels by the stimulus light) and the intensity of the optogenetic illumination. It has been shown that GtACR1 can be a useful inhibitory tool in optogenetic study of Drosophila's visual system by silencing T4/T5 neurons expression.[130] These studies can also be led on intact behaving animals, for instance to probe optomotor response.
Precise temporal control of interventions[]
The currently available optogenetic actuators allow for the accurate temporal control of the required intervention (i.e. inhibition or excitation of the target neurons) with precision routinely going down to the millisecond level.[131] The temporal precision varies, however, across optogenetic actuators,[132] and depends on the frequency and intensity of the stimulation.[94]
Experiments can now be devised where the light used for the intervention is triggered by a particular element of behavior (to inhibit the behavior), a particular unconditioned stimulus (to associate something to that stimulus) or a particular oscillatory event in the brain (to inhibit the event). This kind of approach has already been used in several brain regions:
Hippocampus[]
Sharp waves and ripple complexes (SWRs) are distinct high frequency oscillatory events in the hippocampus thought to play a role in memory formation and consolidation. These events can be readily detected by following the oscillatory cycles of the on-line recorded local field potential. In this way the onset of the event can be used as a trigger signal for a light flash that is guided back into the hippocampus to inhibit neurons specifically during the SWRs and also to optogenetically inhibit the oscillation itself.[133] These kinds of "closed-loop" experiments are useful to study SWR complexes and their role in memory.
Cellular biology/cell signaling pathways[]
Analogously to how natural light-gated ion channels such as channelrhodopsin-2 allows optical control of ion flux, which is especially useful in neuroscience, natural light-controlled signal transduction proteins also allow optical control of biochemical pathways, including both second-messenger generation and protein-protein interactions, which is especially useful in studying cell and developmental biology.[135] In 2002, the first example of using photoproteins from another organism for controlling a biochemical pathway was demonstrated using the light-induced interaction between plant phytochrome and phytochrome-interacting factor (PIF) to control gene transcription in yeast.[3] By fusing phytochrome to a DNA-binding domain and PIF to a transcriptional activation domain, transcriptional activation of genes recognized by the DNA-binding domain could be induced by light.[3] This study anticipated aspects of the later development of optogenetics in the brain, for example, by suggesting that "Directed light delivery by fiber optics has the potential to target selected cells or tissues, even within larger, more-opaque organisms."[3] The literature has been inconsistent as to whether control of cellular biochemistry with photoproteins should be subsumed within the definition of optogenetics, as optogenetics in common usage refers specifically to the control of neuronal firing with opsins,[5][6][7][136] and as control of neuronal firing with opsins postdates and utilizes distinct mechanisms from control of cellular biochemistry with photoproteins.[135]
Photosensitive proteins utilized in various cell signaling pathways[]
In addition to phytochromes, which are found in plants and cyanobacteria, LOV domains(Light-oxygen-voltage-sensing domain) from plants and yeast and cryptochrome domains from plants are other natural photosensory domains that have been used for optical control of biochemical pathways in cells.[137][135] In addition, a synthetic photosensory domain has been engineered from the fluorescent protein Dronpa for optical control of biochemical pathways.[135] In photosensory domains, light absorption is either coupled to a change in protein-protein interactions (in the case of phytochromes, some LOV domains, cryptochromes, and Dronpa mutants) or a conformational change that exposes a linked protein segment or alters the activity of a linked protein domain (in the case of phytochromes and some LOV domains).[135] Light-regulated protein-protein interactions can then be used to recruit proteins to DNA, for example to induce gene transcription or DNA modifications, or to the plasma membrane, for example to activate resident signaling proteins.[134][138][139][140][141][142] CRY2 also clusters when active, so has been fused with signaling domains and subsequently photoactivated to allow for clustering-based activation.[143] The LOV2 domain of Avena sativa(common oat) has been used to expose short peptides or an active protein domain in a light-dependent manner.[144][145][146] Introduction of this LOV domain into another protein can regulate function through light induced peptide disorder.[147] The asLOV2 protein, which optogenetically exposes a peptide, has also been used as a scaffold for several synthetic light induced dimerization and light induced dissociation systems (iLID and LOVTRAP, respectively).[148][149] The systems can be used to control proteins through a protein splitting strategy.[150] Photodissociable Dronpa domains have also been used to cage a protein active site in the dark, uncage it after cyan light illumination, and recage it after violet light illumination.[151]
Temporal control of signal transduction with light[]
The ability to optically control signals for various time durations is being explored to elucidate how cell signaling pathways convert signal duration and response to different outputs.[88] Natural signaling cascades are capable of responding with different outputs to differences in stimulus timing duration and dynamics.[152] For example, treating PC12 cells with epidermal growth factor (EGF, inducing a transient profile of ERK activity) leads to cellular proliferation whereas introduction of nerve growth factor (NGF, inducing a sustained profile of ERK activity) leads to differentiation into neuron-like cells.[153] This behavior was initially characterized using EGF and NGF application, but the finding has been partially replicated with optical inputs.[154] In addition, a rapid negative feedback loop in the RAF-MEK-ERK pathway was discovered using pulsatile activation of a photoswitchable RAF engineered with photodissociable Dronpa domains.[151]
References[]
- ^ Jump up to: a b Deisseroth K, Feng G, Majewska AK, Miesenböck G, Ting A, Schnitzer MJ (October 2006). "Next-generation optical technologies for illuminating genetically targeted brain circuits". The Journal of Neuroscience. 26 (41): 10380–6. doi:10.1523/JNEUROSCI.3863-06.2006. PMC 2820367. PMID 17035522.
- ^ Pathak GP, Vrana JD, Tucker CL (February 2013). "Optogenetic control of cell function using engineered photoreceptors". Biology of the Cell. 105 (2): 59–72. doi:10.1111/boc.201200056. PMC 3552082. PMID 23157573.
- ^ Jump up to: a b c d Shimizu-Sato S, Huq E, Tepperman JM, Quail PH (October 2002). "A light-switchable gene promoter system". Nature Biotechnology. 20 (10): 1041–4. doi:10.1038/nbt734. PMID 12219076. S2CID 24914960.
- ^ Jump up to: a b Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY (August 1997). "Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin". Nature. 388 (6645): 882–7. Bibcode:1997Natur.388..882M. doi:10.1038/42264. PMID 9278050. S2CID 13745050.
- ^ Jump up to: a b Fenno L, Yizhar O, Deisseroth K (2011). "The development and application of optogenetics". Annual Review of Neuroscience. 34: 389–412. doi:10.1146/annurev-neuro-061010-113817. PMC 6699620. PMID 21692661.
- ^ Jump up to: a b "Method of the Year 2010: Optogenetics". Nature Video. 17 December 2010.
- ^ Jump up to: a b c Deisseroth K (20 October 2010). "Optogenetics: Controlling the Brain with Light". Scientific American. Springer Nature America, Inc.
- ^ Lin MZ, Schnitzer MJ (August 2016). "Genetically encoded indicators of neuronal activity". Nature Neuroscience. 19 (9): 1142–53. doi:10.1038/nn.4359. PMC 5557009. PMID 27571193.
- ^ Piatkevich, Kiryl D.; Murdock, Mitchell H.; Subach, Fedor V. (2018). "Advances in Engineering and Application of Optogenetic Indicators for Neuroscience". Applied Sciences. MDPI. 9 (3): 562. doi:10.3390/app9030562.
- ^ Primer on Optogenetics: Pastrana E (2010). "Optogenetics: Controlling cell function with light". Nature Methods. 8 (1): 24–25. doi:10.1038/nmeth.f.323. S2CID 5808517.
Editorial: "Method of the Year 2010". Nature Methods. 8 (1): 1. 2010. doi:10.1038/nmeth.f.321.
Commentary: Deisseroth K (January 2011). "Optogenetics". Nature Methods. 8 (1): 26–9. doi:10.1038/nmeth.f.324. PMC 6814250. PMID 21191368. - ^ Deisseroth K (December 2010). "Insights of the decade. Stepping away from the trees for a look at the forest. Introduction". Science. 330 (6011): 1612–3. Bibcode:2010Sci...330.1612.. doi:10.1126/science.330.6011.1612. PMID 21163985. S2CID 206593135.
- ^ "Method of the Year 2010: Optogenetics". Nature Video. 17 December 2010.
- ^ Crick F (December 1999). "The impact of molecular biology on neuroscience". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 354 (1392): 2021–5. doi:10.1098/rstb.1999.0541. PMC 1692710. PMID 10670022.
- ^ Hoffmann A, Hildebrandt V, Heberle J, Büldt G (September 1994). "Photoactive mitochondria: in vivo transfer of a light-driven proton pump into the inner mitochondrial membrane of Schizosaccharomyces pombe". Proceedings of the National Academy of Sciences of the United States of America. 91 (20): 9367–71. Bibcode:1994PNAS...91.9367H. doi:10.1073/pnas.91.20.9367. PMC 44813. PMID 7937771.
- ^ Fork RL (March 1971). "Laser stimulation of nerve cells in Aplysia". Science. 171 (3974): 907–8. Bibcode:1971Sci...171..907F. doi:10.1126/science.171.3974.907. PMID 5541653. S2CID 484780.
- ^ Zemelman BV, Lee GA, Ng M, Miesenböck G (January 2002). "Selective photostimulation of genetically chARGed neurons". Neuron. 33 (1): 15–22. doi:10.1016/S0896-6273(01)00574-8. PMID 11779476. S2CID 16391269.
- ^ Zemelman BV, Nesnas N, Lee GA, Miesenböck G (February 2003). "Photochemical gating of heterologous ion channels: remote control over genetically designated populations of neurons". Proceedings of the National Academy of Sciences of the United States of America. 100 (3): 1352–7. Bibcode:2003PNAS..100.1352Z. doi:10.1073/pnas.242738899. PMC 298776. PMID 12540832.
- ^ Banghart M, Borges K, Isacoff E, Trauner D, Kramer RH (December 2004). "Light-activated ion channels for remote control of neuronal firing". Nature Neuroscience. 7 (12): 1381–6. doi:10.1038/nn1356. PMC 1447674. PMID 15558062.
- ^ Volgraf M, Gorostiza P, Numano R, Kramer RH, Isacoff EY, Trauner D (January 2006). "Allosteric control of an ionotropic glutamate receptor with an optical switch". Nature Chemical Biology. 2 (1): 47–52. doi:10.1038/nchembio756. PMC 1447676. PMID 16408092.
- ^ Arenkiel BR, Klein ME, Davison IG, Katz LC, Ehlers MD (April 2008). "Genetic control of neuronal activity in mice conditionally expressing TRPV1". Nature Methods. 5 (4): 299–302. doi:10.1038/nmeth.1190. PMC 3127246. PMID 18327266.
- ^ Güler AD, Rainwater A, Parker JG, Jones GL, Argilli E, Arenkiel BR, et al. (March 2012). "Transient activation of specific neurons in mice by selective expression of the capsaicin receptor". Nature Communications. 3: 746. Bibcode:2012NatCo...3..746G. doi:10.1038/ncomms1749. PMC 3592340. PMID 22434189.
- ^ Wang M, Perova Z, Arenkiel BR, Li B (May 2014). "Synaptic modifications in the medial prefrontal cortex in susceptibility and resilience to stress". The Journal of Neuroscience. 34 (22): 7485–92. doi:10.1523/JNEUROSCI.5294-13.2014. PMC 4035514. PMID 24872553.
- ^ Jump up to: a b c Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, et al. (November 2003). "Channelrhodopsin-2, a directly light-gated cation-selective membrane channel". Proceedings of the National Academy of Sciences of the United States of America. 100 (24): 13940–5. Bibcode:2003PNAS..10013940N. doi:10.1073/pnas.1936192100. PMC 283525. PMID 14615590.
- ^ Harz H, Hegemann P (1991-06-06). "Rhodopsin-regulated calcium currents in Chlamydomonas". Nature. 351 (6326): 489–491. Bibcode:1991Natur.351..489H. doi:10.1038/351489a0. S2CID 4309593.
- ^ Nagel G, Ollig D, Fuhrmann M, Kateriya S, Musti AM, Bamberg E, Hegemann P (June 2002). "Channelrhodopsin-1: a light-gated proton channel in green algae". Science. 296 (5577): 2395–8. Bibcode:2002Sci...296.2395N. doi:10.1126/science.1072068. PMID 12089443. S2CID 206506942.
- ^ Deisseroth K (September 2015). "Optogenetics: 10 years of microbial opsins in neuroscience". Nature Neuroscience. 18 (9): 1213–25. doi:10.1038/nn.4091. PMC 4790845. PMID 26308982.
- ^ Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K (September 2005). "Millisecond-timescale, genetically targeted optical control of neural activity". Nature Neuroscience. 8 (9): 1263–8. doi:10.1038/nn1525. PMID 16116447. S2CID 6809511.
- ^ "He may be the rightful inventor of neuroscience's biggest breakthrough in decades. But you've never heard of him". STAT. 1 September 2016. Retrieved 9 February 2020.
- ^ Bi A, Cui J, Ma YP, Olshevskaya E, Pu M, Dizhoor AM, Pan ZH (April 2006). "Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration". Neuron. 50 (1): 23–33. doi:10.1016/j.neuron.2006.02.026. PMC 1459045. PMID 16600853.
- ^ Lima SQ, Miesenböck G (April 2005). "Remote control of behavior through genetically targeted photostimulation of neurons". Cell. 121 (1): 141–52. doi:10.1016/j.cell.2005.02.004. PMID 15820685. S2CID 14608546.
- ^ Jump up to: a b Li X, Gutierrez DV, Hanson MG, Han J, Mark MD, Chiel H, et al. (December 2005). "Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin". Proceedings of the National Academy of Sciences of the United States of America. 102 (49): 17816–21. Bibcode:2005PNAS..10217816L. doi:10.1073/pnas.0509030102. PMC 1292990. PMID 16306259.
- ^ Nagel G, Brauner M, Liewald JF, Adeishvili N, Bamberg E, Gottschalk A (December 2005). "Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses". Current Biology. 15 (24): 2279–84. doi:10.1016/j.cub.2005.11.032. PMID 16360690. S2CID 7036529.
- ^ Tsien JZ, Chen DF, Gerber D, Tom C, Mercer EH, Anderson DJ, et al. (December 1996). "Subregion- and cell type-restricted gene knockout in mouse brain". Cell. 87 (7): 1317–26. doi:10.1016/S0092-8674(00)81826-7. PMID 8980237. S2CID 863399.
- ^ Tsien JZ (2016). "Cre-Lox Neurogenetics: 20 Years of Versatile Applications in Brain Research and Counting…". Frontiers in Genetics. 7: 19. doi:10.3389/fgene.2016.00019. PMC 4759636. PMID 26925095.
- ^ Han X, Boyden ES (2007). "Multiple-Color Optical Activation, Silencing, and Desynchronization of Neural Activity, with Single-Spike Temporal Resolution". PLOS ONE. Public Library of Science. 2 (3): e299. Bibcode:2007PLoSO...2..299H. doi:10.1371/journal.pone.0000299. OCLC 678618519. PMC 1808431. PMID 17375185.
- ^ Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, et al. (April 2007). "Multimodal fast optical interrogation of neural circuitry". Nature. 446 (7136): 633–9. Bibcode:2007Natur.446..633Z. doi:10.1038/nature05744. PMID 17410168. S2CID 4415339.
- ^ Schröder-Lang, Saskia; Schwärzel, Martin; Seifert, Reinhard; Strünker, Timo; Kateriya, Suneel; Looser, Jens; Watanabe, Masakatsu; Kaupp, U Benjamin; Hegemann, Peter; Nagel, Georg (2007). "Fast manipulation of cellular cAMP level by light in vivo". Nature Methods. 4 (1): 39–42. doi:10.1038/nmeth975. ISSN 1548-7091. PMID 17128267. S2CID 10616442.
- ^ Gao, Shiqiang; Nagpal, Jatin; Schneider, Martin W.; Kozjak-Pavlovic, Vera; Nagel, Georg; Gottschalk, Alexander (2015). "Optogenetic manipulation of cGMP in cells and animals by the tightly light-regulated guanylyl-cyclase opsin CyclOp". Nature Communications. 6 (1): 8046. Bibcode:2015NatCo...6.8046G. doi:10.1038/ncomms9046. ISSN 2041-1723. PMC 4569695. PMID 26345128.
- ^ Kerr R, Lev-Ram V, Baird G, Vincent P, Tsien RY, Schafer WR (June 2000). "Optical imaging of calcium transients in neurons and pharyngeal muscle of C. elegans". Neuron. 26 (3): 583–94. doi:10.1016/s0896-6273(00)81196-4. PMID 10896155. S2CID 311998.
- ^ Fiala A, Spall T, Diegelmann S, Eisermann B, Sachse S, Devaud JM, et al. (October 2002). "Genetically expressed cameleon in Drosophila melanogaster is used to visualize olfactory information in projection neurons". Current Biology. 12 (21): 1877–84. doi:10.1016/s0960-9822(02)01239-3. PMID 12419190. S2CID 6312049.
- ^ Higashijima S, Masino MA, Mandel G, Fetcho JR (December 2003). "Imaging neuronal activity during zebrafish behavior with a genetically encoded calcium indicator". Journal of Neurophysiology. 90 (6): 3986–97. doi:10.1152/jn.00576.2003. PMID 12930818. S2CID 2230173.
- ^ Ji G, Feldman ME, Deng KY, Greene KS, Wilson J, Lee JC, et al. (May 2004). "Ca2+-sensing transgenic mice: postsynaptic signaling in smooth muscle". The Journal of Biological Chemistry. 279 (20): 21461–8. doi:10.1074/jbc.M401084200. PMID 14990564.
- ^ Nakai J, Ohkura M, Imoto K (February 2001). "A high signal-to-noise Ca2+ probe composed of a single green fluorescent protein". Nature Biotechnology. 19 (2): 137–41. doi:10.1038/84397. PMID 11175727. S2CID 30254550.
- ^ Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, et al. (July 2013). "Ultrasensitive fluorescent proteins for imaging neuronal activity". Nature. 499 (7458): 295–300. Bibcode:2013Natur.499..295C. doi:10.1038/nature12354. PMC 3777791. PMID 23868258.
- ^ Ninth Annual Wiley Prize in Biomedical Sciences Awarded to Dr. Peter Hegemann, Dr. Georg Nagel, and Dr. Ernst Bamberg (wiley.com)
- ^ Preisträger Archived 2010-07-04 at the Wayback Machine of the Karl Heinz Beckurts Foundation (beckurts-stiftung.de)
- ^ "2010 HFSP Nakasone Award goes to Karl Deisseroth". Human Frontier Science Program (HFSP). Archived from the original on 2014-01-04. Retrieved 2012-07-17.
- ^ "InBev-Baillet Latour International Health Prize" (PDF). Fonds de la Recherche Scientifique - FNRS.
- ^ Louis-Jeantet Prize
- ^ "The Brain Prize 2013". Archived from the original on 4 October 2013. Retrieved 3 October 2013.
- ^ Reiner A, Isacoff EY (October 2013). "The Brain Prize 2013: the optogenetics revolution". Trends in Neurosciences. 36 (10): 557–60. doi:10.1016/j.tins.2013.08.005. PMID 24054067. S2CID 205404606.
- ^ "Kyoto Prize, Inamori Foundation". Kyoto Prize, Inamori Foundation. Retrieved 13 March 2019. "karl-deisseroth-wins-kyoto-prize-for-optogenetics.html".
- ^ "heineken-prize-for-medicine-2020-awarded-to-karl-deisseroth".
- ^ "Rumford Prize Awarded for the Invention and Refinement of Optogenetics". American Academy of Arts & Sciences. Retrieved 2019-03-12.
- ^ Jump up to: a b c Baratta MV, Nakamura S, Dobelis P, Pomrenze MB, Dolzani SD, Cooper DC (2 April 2012). "Optogenetic control of genetically-targeted pyramidal neuron activity in prefrontal cortex" (PDF). Nature Precedings. arXiv:1204.0710. Bibcode:2012arXiv1204.0710B. doi:10.1038/npre.2012.7102.1. S2CID 31641314.
- ^ Husson SJ, Liewald JF, Schultheis C, Stirman JN, Lu H, Gottschalk A (2012). Samuel A (ed.). "Microbial light-activatable proton pumps as neuronal inhibitors to functionally dissect neuronal networks in C. elegans". PLOS ONE. 7 (7): e40937. Bibcode:2012PLoSO...740937H. doi:10.1371/journal.pone.0040937. PMC 3397962. PMID 22815873.

- ^ Liu Y, LeBeouf B, Guo X, Correa PA, Gualberto DG, Lints R, Garcia LR (March 2011). Goodman MB (ed.). "A cholinergic-regulated circuit coordinates the maintenance and bi-stable states of a sensory-motor behavior during Caenorhabditis elegans male copulation". PLOS Genetics. 7 (3): e1001326. doi:10.1371/journal.pgen.1001326. PMC 3053324. PMID 21423722.

- ^ Akemann W, Mutoh H, Perron A, Park YK, Iwamoto Y, Knöpfel T (October 2012). "Imaging neural circuit dynamics with a voltage-sensitive fluorescent protein". Journal of Neurophysiology. 108 (8): 2323–37. doi:10.1152/jn.00452.2012. PMID 22815406. S2CID 14383949.
- ^ Deisseroth K. "Optogenetics: Controlling the Brain with Light [Extended Version]". Scientific American. Retrieved 2016-11-28.
- ^ Beck S, Yu-Strzelczyk J, Pauls D, Constantin OM, Gee CE, Ehmann N, et al. (2018). "Synthetic Light-Activated Ion Channels for Optogenetic Activation and Inhibition". Frontiers in Neuroscience. 12: 643. doi:10.3389/fnins.2018.00643. PMC 6176052. PMID 30333716.
- ^ Sierra YA, Rost B, Oldani S, Schneider-Warme F, Seifert R, Schmitz D, Hegemann P (November 2018). "Potassium channel-based two component optogenetic tool for silencing of excitable cells". Biophysical Journal. 114 (3): 668a. Bibcode:2018BpJ...114..668A. doi:10.1016/j.bpj.2017.11.3607.
- ^ Zhao S, Cunha C, Zhang F, Liu Q, Gloss B, Deisseroth K, et al. (August 2008). "Improved expression of halorhodopsin for light-induced silencing of neuronal activity". Brain Cell Biology. 36 (1–4): 141–54. doi:10.1007/s11068-008-9034-7. PMC 3057022. PMID 18931914.
- ^ Jump up to: a b Gradinaru V, Thompson KR, Deisseroth K (August 2008). "eNpHR: a Natronomonas halorhodopsin enhanced for optogenetic applications". Brain Cell Biology. 36 (1–4): 129–39. doi:10.1007/s11068-008-9027-6. PMC 2588488. PMID 18677566.
- ^ Jump up to: a b c Witten IB, Lin SC, Brodsky M, Prakash R, Diester I, Anikeeva P, et al. (December 2010). "Cholinergic interneurons control local circuit activity and cocaine conditioning". Science. 330 (6011): 1677–81. Bibcode:2010Sci...330.1677W. doi:10.1126/science.1193771. PMC 3142356. PMID 21164015.
- ^ Kim JM, Hwa J, Garriga P, Reeves PJ, RajBhandary UL, Khorana HG (February 2005). "Light-driven activation of beta 2-adrenergic receptor signaling by a chimeric rhodopsin containing the beta 2-adrenergic receptor cytoplasmic loops". Biochemistry. 44 (7): 2284–92. doi:10.1021/bi048328i. PMID 15709741.
- ^ Airan RD, Thompson KR, Fenno LE, Bernstein H, Deisseroth K (April 2009). "Temporally precise in vivo control of intracellular signalling". Nature. 458 (7241): 1025–9. Bibcode:2009Natur.458.1025A. doi:10.1038/nature07926. PMID 19295515. S2CID 4401796.
- ^ Levskaya A, Weiner OD, Lim WA, Voigt CA (October 2009). "Spatiotemporal control of cell signalling using a light-switchable protein interaction". Nature. 461 (7266): 997–1001. Bibcode:2009Natur.461..997L. doi:10.1038/nature08446. PMC 2989900. PMID 19749742.
- ^ Wu YI, Frey D, Lungu OI, Jaehrig A, Schlichting I, Kuhlman B, Hahn KM (September 2009). "A genetically encoded photoactivatable Rac controls the motility of living cells". Nature. 461 (7260): 104–8. Bibcode:2009Natur.461..104W. doi:10.1038/nature08241. PMC 2766670. PMID 19693014.
- ^ Yazawa M, Sadaghiani AM, Hsueh B, Dolmetsch RE (October 2009). "Induction of protein-protein interactions in live cells using light". Nature Biotechnology. 27 (10): 941–5. doi:10.1038/nbt.1569. PMID 19801976. S2CID 205274357.
- ^ Stierl M, Stumpf P, Udwari D, Gueta R, Hagedorn R, Losi A, et al. (January 2011). "Light modulation of cellular cAMP by a small bacterial photoactivated adenylyl cyclase, bPAC, of the soil bacterium Beggiatoa". The Journal of Biological Chemistry. 286 (2): 1181–8. doi:10.1074/jbc.M110.185496. PMC 3020725. PMID 21030594.
- ^ Ryu MH, Moskvin OV, Siltberg-Liberles J, Gomelsky M (December 2010). "Natural and engineered photoactivated nucleotidyl cyclases for optogenetic applications". The Journal of Biological Chemistry. 285 (53): 41501–8. doi:10.1074/jbc.M110.177600. PMC 3009876. PMID 21030591.
- ^ Lerner TN, Ye L, Deisseroth K (March 2016). "Communication in Neural Circuits: Tools, Opportunities, and Challenges". Cell. 164 (6): 1136–1150. doi:10.1016/j.cell.2016.02.027. PMC 5725393. PMID 26967281.
- ^ Aravanis AM, Wang LP, Zhang F, Meltzer LA, Mogri MZ, Schneider MB, Deisseroth K (September 2007). "An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology". Journal of Neural Engineering. 4 (3): S143-56. Bibcode:2007JNEng...4S.143A. doi:10.1088/1741-2560/4/3/S02. PMID 17873414.
- ^ Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L (November 2007). "Neural substrates of awakening probed with optogenetic control of hypocretin neurons". Nature. 450 (7168): 420–4. Bibcode:2007Natur.450..420A. doi:10.1038/nature06310. PMC 6744371. PMID 17943086.
- ^ Jump up to: a b Gradinaru V, Thompson KR, Zhang F, Mogri M, Kay K, Schneider MB, Deisseroth K (December 2007). "Targeting and readout strategies for fast optical neural control in vitro and in vivo". The Journal of Neuroscience. 27 (52): 14231–8. doi:10.1523/JNEUROSCI.3578-07.2007. PMC 6673457. PMID 18160630.
- ^ Damestani Y, Reynolds CL, Szu J, Hsu MS, Kodera Y, Binder DK, et al. (November 2013). "Transparent nanocrystalline yttria-stabilized-zirconia calvarium prosthesis" (PDF). Nanomedicine. 9 (8): 1135–8. doi:10.1016/j.nano.2013.08.002. PMID 23969102. • Explained by Mohan G (September 4, 2013). "A window to the brain? It's here, says UC Riverside team". Los Angeles Times.
- ^ Jump up to: a b Legaria, Alex A.; Licholai, Julia A.; Kravitz, Alexxai V. (January 21, 2021). "Fiber photometry does not reflect spiking activity in the striatum". bioRxiv 10.1101/2021.01.20.427525. doi:10.1101/2021.01.20.427525. S2CID 235967184. Cite journal requires
|journal=(help) - ^ Wentz CT, Bernstein JG, Monahan P, Guerra A, Rodriguez A, Boyden ES (August 2011). "A wirelessly powered and controlled device for optical neural control of freely-behaving animals". Journal of Neural Engineering. 8 (4): 046021. Bibcode:2011JNEng...8d6021W. doi:10.1088/1741-2560/8/4/046021. PMC 3151576. PMID 21701058.
- ^ Jump up to: a b Matarèse BF, Feyen PL, de Mello JC, Benfenati F (2019). "Sub-millisecond Control of Neuronal Firing by Organic Light-Emitting Diodes". Frontiers in Bioengineering and Biotechnology. 7: 278. doi:10.3389/fbioe.2019.00278. PMC 6817475. PMID 31750295.
- ^ Pama EA, Colzato LS, Hommel B (2013-01-01). "Optogenetics as a neuromodulation tool in cognitive neuroscience". Frontiers in Psychology. 4: 610. doi:10.3389/fpsyg.2013.00610. PMC 3764402. PMID 24046763.
- ^ Zhang, Feng; Gradinaru, Viviana; Adamantidis, Antoine R; Durand, Remy; Airan, Raag D; de Lecea, Luis; Deisseroth, Karl (March 2010). "Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures". Nature Protocols. 5 (3): 439–456. doi:10.1038/nprot.2009.226. PMC 4503465. PMID 20203662.
- ^ Zeng, Hongkui; Madisen, Linda (2012-09-05). "Mouse transgenic approaches in optogenetics". Prog. Brain Res. Progress in Brain Research. 196: 193–213. doi:10.1016/B978-0-444-59426-6.00010-0. ISBN 9780444594266. PMC 3433654. PMID 22341327.
- ^ Warden MR, Cardin JA, Deisseroth K (July 2014). "Optical neural interfaces". Annual Review of Biomedical Engineering. 16: 103–29. doi:10.1146/annurev-bioeng-071813-104733. PMC 4163158. PMID 25014785.
- ^ Guru A, Post RJ, Ho YY, Warden MR (July 2015). "Making Sense of Optogenetics". The International Journal of Neuropsychopharmacology. 18 (11): pyv079. doi:10.1093/ijnp/pyv079. PMC 4756725. PMID 26209858.
- ^ "The Evolution in Freely-Behaving Imaging and Optogenetics Technology". OASIS Implant. Mightex. Retrieved 2021-06-03.
- ^ Kim CK, Yang SJ, Pichamoorthy N, Young NP, Kauvar I, Jennings JH, Lerner TN, Berndt A, Lee SY, Ramakrishnan C, Davidson TJ, Inoue M, Bito H, Deisseroth K (April 2016). "Simultaneous fast measurement of circuit dynamics at multiple sites across the mammalian brain". Nature Methods. 13 (4): 325–328. doi:10.1038/nmeth.3770. PMC 4100551. PMID 24784819.
- ^ Cui G, Jun SB, Jin X, Luo G, Pham MD, Lovinger DM, Vogel SS, Costa RM (June 2014). "Deep brain optical measurements of cell type–specific neural activity in behaving mice". Nature Protocols. 9 (6): 1213–1228. doi:10.1038/nprot.2014.080. PMC 4100551. PMID 24784819.
- ^ Jump up to: a b Tischer D, Weiner OD (August 2014). "Illuminating cell signalling with optogenetic tools". Nature Reviews. Molecular Cell Biology. 15 (8): 551–8. doi:10.1038/nrm3837. PMC 4145075. PMID 25027655.
- ^ Jump up to: a b c d e Zalocusky KA, Fenno LE, Deisseroth K (2013). "Current Challenges in Optogenetics". Society for Neuroscience.
- ^ Heitmann S, Rule M, Truccolo W, Ermentrout B (January 2017). "Optogenetic Stimulation Shifts the Excitability of Cerebral Cortex from Type I to Type II: Oscillation Onset and Wave Propagation". PLOS Computational Biology. 13 (1): e1005349. Bibcode:2017PLSCB..13E5349H. doi:10.1371/journal.pcbi.1005349. PMC 5295702. PMID 28118355.
- ^ Lu Y, Truccolo W, Wagner FB, Vargas-Irwin CE, Ozden I, Zimmermann JB, et al. (June 2015). "Optogenetically induced spatiotemporal gamma oscillations and neuronal spiking activity in primate motor cortex". Journal of Neurophysiology. 113 (10): 3574–87. doi:10.1152/jn.00792.2014. PMC 4461886. PMID 25761956.
- ^ Jump up to: a b Leergaard TB, Hilgetag CC, Sporns O (2012-05-01). "Mapping the connectome: multi-level analysis of brain connectivity". Frontiers in Neuroinformatics. 6: 14. doi:10.3389/fninf.2012.00014. PMC 3340894. PMID 22557964.
- ^ Penzkofer A, Hegemann P, Kateriya S (2018). "Organic dyes in optogenetics". In Duarte FJ (ed.). Organic Lasers and Organic Photonics. London: Institute of Physics. pp. 13–1 to 13–114. ISBN 978-0-7503-1570-8.
- ^ Jump up to: a b Luboeinski J, Tchumatchenko T (September 2020). "Nonlinear response characteristics of neural networks and single neurons undergoing optogenetic excitation". Network Neuroscience. 4 (3): 852–870. doi:10.1162/netn_a_00154. PMC 7888483. PMID 33615093.
- ^ "PyRhO: a virtual optogenetics laboratory". GitHub.
- ^ "Simulation tool for neural networks and single neurons with light-sensitive channels". GitHub.
- ^ Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC (July 2010). "Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry". Nature. 466 (7306): 622–6. Bibcode:2010Natur.466..622K. doi:10.1038/nature09159. PMC 3552484. PMID 20613723.
- ^ Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K (April 2009). "Optical deconstruction of parkinsonian neural circuitry". Science. 324 (5925): 354–9. Bibcode:2009Sci...324..354G. CiteSeerX 10.1.1.368.668. doi:10.1126/science.1167093. PMC 6744370. PMID 19299587.
- ^ Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, et al. (June 2009). "Driving fast-spiking cells induces gamma rhythm and controls sensory responses". Nature. 459 (7247): 663–7. Bibcode:2009Natur.459..663C. doi:10.1038/nature08002. PMC 3655711. PMID 19396156.
- ^ Sohal VS, Zhang F, Yizhar O, Deisseroth K (June 2009). "Parvalbumin neurons and gamma rhythms enhance cortical circuit performance". Nature. 459 (7247): 698–702. Bibcode:2009Natur.459..698S. doi:10.1038/nature07991. PMC 3969859. PMID 19396159.
- ^ Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K (May 2009). "Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning". Science. 324 (5930): 1080–4. Bibcode:2009Sci...324.1080T. doi:10.1126/science.1168878. PMC 5262197. PMID 19389999.
- ^ Zimmer, Carl (24 May 2021). "Scientists Partially Restored a Blind Man's Sight With New Gene Therapy". The New York Times. Retrieved 25 May 2021.
- ^ Sahel, José-Alain; Boulanger-Scemama, Elise; Pagot, Chloé; Arleo, Angelo; Galluppi, Francesco; Martel, Joseph N.; Esposti, Simona Degli; Delaux, Alexandre; de Saint Aubert, Jean-Baptiste; de Montleau, Caroline; Gutman, Emmanuel; Audo, Isabelle; Duebel, Jens; Picaud, Serge; Dalkara, Deniz; Blouin, Laure; Taiel, Magali; Roska, Botond (2021-05-24). "Partial recovery of visual function in a blind patient after optogenetic therapy". Nature Medicine. 27 (7): 1223–1229. doi:10.1038/s41591-021-01351-4. ISSN 1546-170X. PMID 34031601.
- ^ Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, et al. (November 2010). "Genetic dissection of an amygdala microcircuit that gates conditioned fear". Nature. 468 (7321): 270–6. Bibcode:2010Natur.468..270H. doi:10.1038/nature09553. PMC 3597095. PMID 21068836.
- ^ Johansen JP, Hamanaka H, Monfils MH, Behnia R, Deisseroth K, Blair HT, LeDoux JE (July 2010). "Optical activation of lateral amygdala pyramidal cells instructs associative fear learning". Proceedings of the National Academy of Sciences of the United States of America. 107 (28): 12692–7. Bibcode:2010PNAS..10712692J. doi:10.1073/pnas.1002418107. PMC 2906568. PMID 20615999.
- ^ Jasnow AM, Ehrlich DE, Choi DC, Dabrowska J, Bowers ME, McCullough KM, et al. (June 2013). "Thy1-expressing neurons in the basolateral amygdala may mediate fear inhibition". The Journal of Neuroscience. 33 (25): 10396–404. doi:10.1523/JNEUROSCI.5539-12.2013. PMC 3685835. PMID 23785152.
- ^ Dias BG, Banerjee SB, Goodman JV, Ressler KJ (June 2013). "Towards new approaches to disorders of fear and anxiety". Current Opinion in Neurobiology. 23 (3): 346–52. doi:10.1016/j.conb.2013.01.013. PMC 3672317. PMID 23402950.
- ^ Karalis N, Dejean C, Chaudun F, Khoder S, Rozeske RR, Wurtz H, et al. (April 2016). "4-Hz oscillations synchronize prefrontal-amygdala circuits during fear behavior". Nature Neuroscience. 19 (4): 605–12. doi:10.1038/nn.4251. PMC 4843971. PMID 26878674.
- ^ Shusterman R, Smear MC, Koulakov AA, Rinberg D (July 2011). "Precise olfactory responses tile the sniff cycle". Nature Neuroscience. 14 (8): 1039–44. doi:10.1038/nn.2877. PMID 21765422. S2CID 5194595.
- ^ Smith RS, Hu R, DeSouza A, Eberly CL, Krahe K, Chan W, Araneda RC (July 2015). "Differential Muscarinic Modulation in the Olfactory Bulb". The Journal of Neuroscience. 35 (30): 10773–85. doi:10.1523/JNEUROSCI.0099-15.2015. PMC 4518052. PMID 26224860.
- ^ Patterson MA, Lagier S, Carleton A (August 2013). "Odor representations in the olfactory bulb evolve after the first breath and persist as an odor afterimage". Proceedings of the National Academy of Sciences of the United States of America. 110 (35): E3340-9. Bibcode:2013PNAS..110E3340P. doi:10.1073/pnas.1303873110. PMC 3761593. PMID 23918364.
- ^ Tecuapetla F, Patel JC, Xenias H, English D, Tadros I, Shah F, et al. (May 2010). "Glutamatergic signaling by mesolimbic dopamine neurons in the nucleus accumbens". The Journal of Neuroscience. 30 (20): 7105–10. doi:10.1523/JNEUROSCI.0265-10.2010. PMC 3842465. PMID 20484653.
- ^ Cela E, McFarlan AR, Chung AJ, Wang T, Chierzi S, Murai KK, Sjöström PJ (March 2019). "An Optogenetic Kindling Model of Neocortical Epilepsy". Scientific Reports. 9 (1): 5236. Bibcode:2019NatSR...9.5236C. doi:10.1038/s41598-019-41533-2. PMC 6437216. PMID 30918286.
- ^ Jump up to: a b Ryu, Brendan; Nagappan, Shivathmihai; Santos-Valencia, Fernando; Lee, Psyche; Rodriguez, Erica; Lackie, Meredith; Takatoh, Jun; Franks, Kevin M. (April 2021). "Chronic loss of inhibition in piriform cortex following brief, daily optogenetic stimulation". Cell Reports. 35 (3): 109001. doi:10.1016/j.celrep.2021.109001. ISSN 2211-1247. PMC 8102022. PMID 33882304.
- ^ Bingen BO, Engels MC, Schalij MJ, Jangsangthong W, Neshati Z, Feola I, et al. (October 2014). "Light-induced termination of spiral wave arrhythmias by optogenetic engineering of atrial cardiomyocytes". Cardiovascular Research. 104 (1): 194–205. doi:10.1093/cvr/cvu179. PMID 25082848.
- ^ Nussinovitch U, Gepstein L (July 2015). "Optogenetics for in vivo cardiac pacing and resynchronization therapies". Nature Biotechnology. 33 (7): 750–4. doi:10.1038/nbt.3268. PMID 26098449. S2CID 1794556.
- ^ Nyns EC, Kip A, Bart CI, Plomp JJ, Zeppenfeld K, Schalij MJ, et al. (July 2017). "Optogenetic termination of ventricular arrhythmias in the whole heart: towards biological cardiac rhythm management". European Heart Journal. 38 (27): 2132–2136. doi:10.1093/eurheartj/ehw574. PMC 5837774. PMID 28011703.
- ^ Bruegmann T, Boyle PM, Vogt CC, Karathanos TV, Arevalo HJ, Fleischmann BK, et al. (October 2016). "Optogenetic defibrillation terminates ventricular arrhythmia in mouse hearts and human simulations". The Journal of Clinical Investigation. 126 (10): 3894–3904. doi:10.1172/JCI88950. PMC 5096832. PMID 27617859.
- ^ Crocini C, Ferrantini C, Coppini R, Scardigli M, Yan P, Loew LM, et al. (October 2016). "Optogenetics design of mechanistically-based stimulation patterns for cardiac defibrillation". Scientific Reports. 6: 35628. Bibcode:2016NatSR...635628C. doi:10.1038/srep35628. PMC 5066272. PMID 27748433.
- ^ Hernandez VH, Gehrt A, Reuter K, Jing Z, Jeschke M, Mendoza Schulz A, et al. (March 2014). "Optogenetic stimulation of the auditory pathway". The Journal of Clinical Investigation. 124 (3): 1114–29. doi:10.1172/JCI69050. PMC 3934189. PMID 24509078.
- ^ Keppeler D, Merino RM, Lopez de la Morena D, Bali B, Huet AT, Gehrt A, et al. (December 2018). "Ultrafast optogenetic stimulation of the auditory pathway by targeting-optimized Chronos". The EMBO Journal. 37 (24): e99649. doi:10.15252/embj.201899649. PMC 6293277. PMID 30396994.
- ^ Mager T, Lopez de la Morena D, Senn V, Schlotte J, D Errico A, Feldbauer K, et al. (May 2018). "High frequency neural spiking and auditory signaling by ultrafast red-shifted optogenetics". Nature Communications. 9 (1): 1750. Bibcode:2018NatCo...9.1750M. doi:10.1038/s41467-018-04146-3. PMC 5931537. PMID 29717130.
- ^ "Engineering long-wavelength light-driven ion channels to hear the light. Atlas of Science". Retrieved 7 November 2019.
- ^ Moser T (October 2015). "Optogenetic stimulation of the auditory pathway for research and future prosthetics". Current Opinion in Neurobiology. 34: 29–36. doi:10.1016/j.conb.2015.01.004. PMID 25637880. S2CID 35199775.
- ^ Lin JY, Knutsen PM, Muller A, Kleinfeld D, Tsien RY (October 2013). "ReaChR: a red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation". Nature Neuroscience. 16 (10): 1499–508. doi:10.1038/nn.3502. PMC 3793847. PMID 23995068.
- ^ Matthews GA, Nieh EH, Vander Weele CM, Halbert SA, Pradhan RV, Yosafat AS, et al. (February 2016). "Dorsal Raphe Dopamine Neurons Represent the Experience of Social Isolation". Cell. 164 (4): 617–31. doi:10.1016/j.cell.2015.12.040. PMC 4752823. PMID 26871628.
- ^ Klapoetke NC, Murata Y, Kim SS, Pulver SR, Birdsey-Benson A, Cho YK, et al. (March 2014). "Independent optical excitation of distinct neural populations". Nature Methods. 11 (3): 338–46. doi:10.1038/nmeth.2836. PMC 3943671. PMID 24509633.
- ^ Berndt A, Yizhar O, Gunaydin LA, Hegemann P, Deisseroth K (February 2009). "Bi-stable neural state switches". Nature Neuroscience. 12 (2): 229–34. doi:10.1038/nn.2247. PMID 19079251. S2CID 15125498.
- ^ Govorunova EG, Sineshchekov OA, Janz R, Liu X, Spudich JL (August 2015). "NEUROSCIENCE. Natural light-gated anion channels: A family of microbial rhodopsins for advanced optogenetics". Science. 349 (6248): 647–50. doi:10.1126/science.aaa7484. PMC 4764398. PMID 26113638.
- ^ Mauss AS, Busch C, Borst A (October 2017). "Optogenetic Neuronal Silencing in Drosophila during Visual Processing". Scientific Reports. 7 (1): 13823. Bibcode:2017NatSR...713823M. doi:10.1038/s41598-017-14076-7. PMC 5653863. PMID 29061981.
- ^ Solari N, Sviatkó K, Laszlovszky T, Hegedüs P, Hangya B (May 2018). "Open source tools for temporally controlled rodent behavior suitable for electrophysiology and optogenetic manipulations". Frontiers in Systems Neuroscience. 12: 18. doi:10.3389/fnsys.2018.00018. PMC 5962774. PMID 29867383.
- ^ Lin JY (December 2010). "A user's guide to channelrhodopsin variants: Features, limitations and future developments". Experimental Physiology. 96 (1): 19–25. doi:10.1113/expphysiol.2009.051961. PMC 2995811. PMID 20621963.
- ^ Kovács KA, O'Neill J, Schoenenberger P, Penttonen M, Ranguel Guerrero DK, Csicsvari J (19 Nov 2016). "Optogenetically Blocking Sharp Wave Ripple Events in Sleep Does Not Interfere with the Formation of Stable Spatial Representation in the CA1 Area of the Hippocampus". PLOS ONE. 11 (10): e0164675. Bibcode:2016PLoSO..1164675K. doi:10.1371/journal.pone.0164675. PMC 5070819. PMID 27760158.
- ^ Jump up to: a b Valon L, Marín-Llauradó A, Wyatt T, Charras G, Trepat X (February 2017). "Optogenetic control of cellular forces and mechanotransduction". Nature Communications. 8: 14396. Bibcode:2017NatCo...814396V. doi:10.1038/ncomms14396. PMC 5309899. PMID 28186127.
- ^ Jump up to: a b c d e Khamo JS, Krishnamurthy VV, Sharum SR, Mondal P, Zhang K (October 2017). "Applications of Optobiology in Intact Cells and Multicellular Organisms". Journal of Molecular Biology. 429 (20): 2999–3017. doi:10.1016/j.jmb.2017.08.015. PMID 28882542.
- ^ "optogenetics - Search Results". PubMed. Retrieved 2020-02-29.
- ^ Wittmann T, Dema A, van Haren J (May 2020). "Lights, cytoskeleton, action: Optogenetic control of cell dynamics". Current Opinion in Cell Biology. Elsevier Ltd. 66: 1–10. doi:10.1016/j.ceb.2020.03.003. PMC 7577957. PMID 32371345.
- ^ Konermann S, Brigham MD, Trevino A, Hsu PD, Heidenreich M, Cong L, et al. (August 2013). "Optical control of mammalian endogenous transcription and epigenetic states". Nature. 500 (7463): 472–476. Bibcode:2013Natur.500..472K. doi:10.1038/nature12466. PMC 3856241. PMID 23877069.
- ^ Leung DW, Otomo C, Chory J, Rosen MK (September 2008). "Genetically encoded photoswitching of actin assembly through the Cdc42-WASP-Arp2/3 complex pathway". Proceedings of the National Academy of Sciences of the United States of America. 105 (35): 12797–802. Bibcode:2008PNAS..10512797L. doi:10.1073/pnas.0801232105. PMC 2525560. PMID 18728185.
- ^ Toettcher JE, Gong D, Lim WA, Weiner OD (September 2011). "Light-based feedback for controlling intracellular signaling dynamics". Nature Methods. 8 (10): 837–9. doi:10.1038/nmeth.1700. PMC 3184382. PMID 21909100.
- ^ Strickland D, Lin Y, Wagner E, Hope CM, Zayner J, Antoniou C, et al. (March 2012). "TULIPs: tunable, light-controlled interacting protein tags for cell biology". Nature Methods. 9 (4): 379–84. doi:10.1038/nmeth.1904. PMC 3444151. PMID 22388287.
- ^ Idevall-Hagren O, Dickson EJ, Hille B, Toomre DK, De Camilli P (August 2012). "Optogenetic control of phosphoinositide metabolism". Proceedings of the National Academy of Sciences of the United States of America. 109 (35): E2316-23. Bibcode:2012PNAS..109E2316I. doi:10.1073/pnas.1211305109. PMC 3435206. PMID 22847441.
- ^ Bugaj LJ, Choksi AT, Mesuda CK, Kane RS, Schaffer DV (March 2013). "Optogenetic protein clustering and signaling activation in mammalian cells". Nature Methods. 10 (3): 249–52. doi:10.1038/nmeth.2360. PMID 23377377. S2CID 8737019.
- ^ Lungu OI, Hallett RA, Choi EJ, Aiken MJ, Hahn KM, Kuhlman B (April 2012). "Designing photoswitchable peptides using the AsLOV2 domain". Chemistry & Biology. 19 (4): 507–17. doi:10.1016/j.chembiol.2012.02.006. PMC 3334866. PMID 22520757.
- ^ Wu YI, Frey D, Lungu OI, Jaehrig A, Schlichting I, Kuhlman B, Hahn KM (September 2009). "A genetically encoded photoactivatable Rac controls the motility of living cells". Nature. 461 (7260): 104–8. Bibcode:2009Natur.461..104W. doi:10.1038/nature08241. PMC 2766670. PMID 19693014.
- ^ Smart AD, Pache RA, Thomsen ND, Kortemme T, Davis GW, Wells JA (September 2017). "Engineering a light-activated caspase-3 for precise ablation of neurons in vivo". Proceedings of the National Academy of Sciences of the United States of America. 114 (39): E8174–E8183. doi:10.1073/pnas.1705064114. PMC 5625904. PMID 28893998.
- ^ Dagliyan O, Tarnawski M, Chu PH, Shirvanyants D, Schlichting I, Dokholyan NV, Hahn KM (December 2016). "Engineering extrinsic disorder to control protein activity in living cells". Science. 354 (6318): 1441–1444. Bibcode:2016Sci...354.1441D. doi:10.1126/science.aah3404. PMC 5362825. PMID 27980211.
- ^ Guntas G, Hallett RA, Zimmerman SP, Williams T, Yumerefendi H, Bear JE, Kuhlman B (January 2015). "Engineering an improved light-induced dimer (iLID) for controlling the localization and activity of signaling proteins". Proceedings of the National Academy of Sciences of the United States of America. 112 (1): 112–7. Bibcode:2015PNAS..112..112G. doi:10.1073/pnas.1417910112. PMC 4291625. PMID 25535392.
- ^ Wang H, Vilela M, Winkler A, Tarnawski M, Schlichting I, Yumerefendi H, et al. (September 2016). "LOVTRAP: an optogenetic system for photoinduced protein dissociation". Nature Methods. 13 (9): 755–8. doi:10.1038/nmeth.3926. PMC 5137947. PMID 27427858.
- ^ van Haren J, Charafeddine RA, Ettinger A, Wang H, Hahn KM, Wittmann T (March 2018). "Local control of intracellular microtubule dynamics by EB1 photodissociation". Nature Cell Biology. Nature Research. 20 (3): 252–261. doi:10.1038/s41556-017-0028-5. PMC 5826794. PMID 29379139.
- ^ Jump up to: a b Zhou XX, Chung HK, Lam AJ, Lin MZ (November 2012). "Optical control of protein activity by fluorescent protein domains". Science. 338 (6108): 810–4. Bibcode:2012Sci...338..810Z. doi:10.1126/science.1226854. PMC 3702057. PMID 23139335.
- ^ Purvis JE, Lahav G (February 2013). "Encoding and decoding cellular information through signaling dynamics". Cell. 152 (5): 945–56. doi:10.1016/j.cell.2013.02.005. PMC 3707615. PMID 23452846.
- ^ Santos SD, Verveer PJ, Bastiaens PI (March 2007). "Growth factor-induced MAPK network topology shapes Erk response determining PC-12 cell fate". Nature Cell Biology. 9 (3): 324–30. doi:10.1038/ncb1543. PMID 17310240. S2CID 31709706.
- ^ Toettcher JE, Weiner OD, Lim WA (December 2013). "Using optogenetics to interrogate the dynamic control of signal transmission by the Ras/Erk module". Cell. 155 (6): 1422–34. doi:10.1016/j.cell.2013.11.004. PMC 3925772. PMID 24315106.
Further reading[]
- Appasani K (2017). Optogenetics: from neuronal function to mapping and disease biology. Cambridge, UK: Cambridge University Press. ISBN 978-1-107-05301-4.
- Banerjee S, Mitra D (January 2020). "Structural Basis of Design and Engineering for Advanced Plant Optogenetics". Trends in Plant Science. 25 (1): 35–65. doi:10.1016/j.tplants.2019.10.002. PMID 31699521.
- Hu W, Li Q, Li B, Ma K, Zhang C, Fu X (January 2020). "Optogenetics sheds new light on tissue engineering and regenerative medicine". Biomaterials. 227: 119546. doi:10.1016/j.biomaterials.2019.119546. PMID 31655444. S2CID 204918731.
- Jarrin S, Finn DP (October 2019). "Optogenetics and its application in pain and anxiety research". Neuroscience and Biobehavioral Reviews. 105: 200–211. doi:10.1016/j.neubiorev.2019.08.007. PMID 31421140. S2CID 199577276.
- Johnson HE, Toettcher JE (August 2018). "Illuminating developmental biology with cellular optogenetics". Current Opinion in Biotechnology. 52: 42–48. doi:10.1016/j.copbio.2018.02.003. PMC 6082700. PMID 29505976.
- Krueger D, Izquierdo E, Viswanathan R, Hartmann J, Pallares Cartes C, De Renzis S (October 2019). "Principles and applications of optogenetics in developmental biology". Development. Cambridge, England. 146 (20): dev175067. doi:10.1242/dev.175067. PMC 6914371. PMID 31641044.
- Losi A, Gardner KH, Möglich A (November 2018). "Blue-Light Receptors for Optogenetics". Chemical Reviews. 118 (21): 10659–10709. doi:10.1021/acs.chemrev.8b00163. PMC 6500593. PMID 29984995.
- Vriz S, Ozawa T (September 2018). Optogenetics: light-driven actuators and light-emitting sensors in cell biology. Comprehensive Series in Photochemistry and Photobiology. 18. London: Royal Society of Chemistry. ISBN 978-1-78801-237-9.
- Wittmann T, Dema A, van Haren J (May 2020). "Lights, cytoskeleton, action: Optogenetic control of cell dynamics". Current Opinion in Cell Biology, Themed Issue : Cell Dynamics. Elsevier Ltd. 66: 1–10. doi:10.1016/j.ceb.2020.03.003. PMC 7577957. PMID 32371345.
External links[]
- "Optogenetics: shedding light on the brain's secrets". Scientifica.
- Neuroscience
- Biological techniques and tools
- Cybernetics
- Control theory
- Brain–computer interfacing
- Neuroprosthetics
- Neural engineering
- Optics