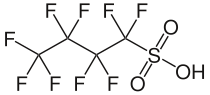
Perfluorobutanesulfonic acid
| |
| Names | |
|---|---|
| Preferred IUPAC name
1,1,2,2,3,3,4,4,4-Nonafluorobutane-1-sulfonic acid | |
| Other names
FC-98
Nonaflate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.006.176 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 3094, 3265 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4HF9O3S | |
| Molar mass | 300.10 g/mol |
| Melting point | 76 to 84 °C (169 to 183 °F; 349 to 357 K) [1] |
| Boiling point | 211 °C (412 °F; 484 K)[2] |
| Hazards | |
| GHS labelling: | |
  [3] [3]
| |
Signal word
|
Danger |
| H302, H314 | |
| P280, P305+P351+P338, P310[3] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Perfluorobutanesulfonic acid (PFBS) is a chemical compound with a four carbon fluorocarbon chain and a sulfonic acid functional group. PFBS can be in the form of a colorless liquid or a corrosive solid.[4] As an anion it functions as a stable fluorosurfactant because of the strength of carbon–fluorine bonds.
Since June 2003, 3M has used PFBS as a replacement for the persistent, toxic, and bioaccumulative perfluorooctanesulfonic acid (PFOS) in its Scotchgard stain repellents.[5] 3M markets surfactant with PFBS in two fluorosurfactants.[6]
Safety[]
PFBS has a half-life of a little over one month in humans, much shorter than PFOS with 5.4 years.[7] PFBS is persistent in the environment. Studies have not yet been specifically conducted to determine safety in humans.
The ECHA decision adding PFBS and its salts to the REACH Regulation Candidate List of Substances of Very High Concern states:
“The combined intrinsic properties justifying the inclusion as a substance for which there is scientific evidence of probable serious effects to human health and the environment which give rise to an equivalent level of concern are the following: very high persistence, high mobility in water and soil, high potential for long-range transport, and difficulty of remediation and water purification as well as moderate bioaccumulation in humans. The observed probable serious effects for human health and the environment are thyroid hormonal disturbances and reproductive toxicity seen in rodents, and effects on liver, kidney and haematological system in rats, hormonal disturbances and effects on reproduction in marine medaka fish and effects on expression of hormone receptors in tadpoles. Together, these elements lead to a very high potential for irreversible effects.”[8]
Legislation and Regulation[]
European Union[]
On 2020-01-16, PFBS and its salts were added to the REACH Regulation Candidate List of Substances of Very High Concern (SVHCs) on the grounds of "Equivalent level of concern having probable serious effects to human health (Article 57(f) - human health)" and "Equivalent level of concern having probable serious effects to the environment (Article 57(f) - environment)".[8]
United States[]
There is no national standard for PFBS. However, several states have proposed regulating PFBS in drinking water including Michigan either as contamination standards, guidance or health advisories.[9]
See also[]
- PFOA
- PFNA
- FBSA
- Perfluorinated alkylated substances (PFAS)
- Timeline of events related to per- and polyfluoroalkyl substances (PFAS)
References[]
- ^ "Nonafluorobutanesulphonic acid – 59933-66-3 Catalog of Chemical Suppliers". Retrieved 16 January 2009.
- ^ Perfluorobutanesulfonic acid in the ChemIDplus database
- ^ a b Sigma-Aldrich Co., Nonafluorobutane-1-sulfonic acid. Retrieved on 15 January 2018.
- ^ "Perfluorobutanesulfonic acid". PubChem. NIH. Retrieved 10 August 2021.
- ^ Ullah, Aziz (October 2006). "The Fluorochemical Dilemma: What the PFOS/PFOA fuss is all about" (PDF). Cleaning & Restoration. Retrieved 16 January 2009.
- ^ Renner R (January 2006). "The long and the short of perfluorinated replacements". Environ. Sci. Technol. 40 (1): 12–3. doi:10.1021/es062612a. PMID 16433328.
- ^ Betts KS (May 2007). "Perfluoroalkyl acids: what is the evidence telling us?". Environ. Health Perspect. 115 (5): A250–6. doi:10.1289/ehp.115-a250. PMC 1867999. PMID 17520044.
- ^ a b Schilliger-Musset, Christel (2020-01-16). "Inclusion of substances of very high concern in the Candidate List for eventual inclusion in Annex XIV" (PDF). European Chemicals Agency (ECHA).
- ^ "State-by-State Regulation of PFAS Substances in Drinking Water". Bryan Cave Leighton Paisner LLP. January 22, 2021. Retrieved 11 August 2021.
- Anionic surfactants
- Perfluorosulfonic acids