Perfluorocycloalkene
A perfluorocycloalkene (PFCA) fluorocarbon structure with a cycloalkene core. PFCAs have shown reactivity with a wide variety of nucleophiles including phenoxides, alkoxides, organometallic, amines, thiols, and azoles.[1] They or their derivatives are reported to have nonlinear optical activity,[2] and be useful as lubricants,[3] etching agents,[4] components of fuel cells,[5] low dielectric materials, and super hydrophobic and oleophobic coatings.[6]
- Examples of perfluorocycloalkenes

Tetrafluorocyclopropene
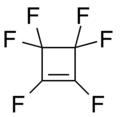
Hexafluorocyclobutene

Octafluorocyclopentene

Decafluorocyclohexene
Reactivity[]
Derivatization of these PFCA rings via displacement of fluorine atoms with nucleophiles occurs through an addition-elimination reaction in the presence of a base. Attack of the nucleophile on the PFCA ring generates a carbanion which can eliminate a fluoride ion, resulting in vinyl substituted and allyl substituted products (Scheme 1). The ratio of vinylic to allylic products depends on the ring size, reaction conditions and nucleophile.[1][7]
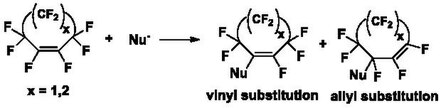
Under favorable conditions, a good nucleophile can replace all the fluorine atoms on PFCA ring (Scheme 2).[8]
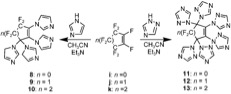
PFCAs have a huge potential to be used as a monomer to produce a variety of polymers. The first time, Smith et al. showed the polycondensation of bisphenols with PFCAs. A unique class of aromatic ether polymers containing perfluorocyclopentenyl (PFCP) enchainment was prepared from the simple of commercial available bisphenols and octafluorocyclopentene (OFCP) in the presence of triethylamine (Scheme 3 and 4).[6][9]


Smith et al. further extended his recently published work on perfluorocyclopentenyl (PFCP) aryl ether polymers and perfuorocycloalkenyl (PFCA) aryl ether monomers, and reported the synthesis of a new class of fluoropolymers, namely, perfluorocyclohexenyl (PFCH) aryl ether polymers, via step-growth polycondensation of commercial bisphenols and decafluorocycloalkene (DFCH) in the presence of triethylamine (Scheme 5).[7][10]

References[]
- ^ a b c d Wigglesworth, Tony J.; Sud, David; Norsten, Tyler B.; Lekhi, Vikram S.; Branda, Neil R. (2005). "Chiral Discrimination in Photochromic Helicenes". Journal of the American Chemical Society. 127 (20): 7272–3. doi:10.1021/ja050190j. PMID 15898750.
- ^ Matsui, Masaki; Tsuge, Michinori; Funabiki, Kazumasa; Shibata, Katsuyoshi; Muramatsu, Hiroshige; Hirota, Kazuo; Hosoda, Masahiro; Tai, Kazuo; Shiozaki, Hisayoshi; Kim, Misa; Nakatsu, Kazumi (1999). "Synthesis of azo chromophores containing a perfluorocyclo-alkenyl moiety and their second-order optical nonlinearity". Journal of Fluorine Chemistry. 97 (1–2): 207–12. doi:10.1016/S0022-1139(99)00050-0.
- ^ "Organometallic Derivatives Of Perfluorocycloalkenes - Minnesota Mining Mfg Co,Us". Freepatentsonline.com. 1971-08-25. Retrieved 2017-01-05.
- ^ Takahashi, Kazuo; Itoh, Atsushi; Nakamura, Toshihiro; Tachibana, Kunihide (2000). "Radical kinetics for polymer film deposition in fluorocarbon (C4F8, C3F6 and C5F8) plasmas". Thin Solid Films. 374 (2): 303–10. Bibcode:2000TSF...374..303T. doi:10.1016/S0040-6090(00)01160-3.
- ^ "Patent US20140162173 - Sulfonated perfluorocyclopentenyl polymers and uses thereof - Google Patents". Google.com. 2013-10-09. Retrieved 2017-01-05.
- ^ a b Sharma, Babloo; Verma, Rajneesh; Baur, Cary; Bykova, Julia; Mabry, Joseph M.; Smith, Dennis W. (2013). "Ultra low dielectric, self-cleansing and highly oleophobic POSS-PFCP aryl ether polymer composites". Journal of Materials Chemistry C. 1 (43): 7222–7. doi:10.1039/C3TC31161A.
- ^ a b c Sharma, Babloo; Faisal, Mohammad; Liff, Shawna M.; Smith, Dennis W. (2014). "Triarylamine-enchained semifluorinated perfluorocycloalkenyl (PFCA) aryl ether polymers". Applied Petrochemical Research. 5: 35. doi:10.1007/s13203-014-0063-0.
- ^ a b Garg, Sonali; Twamley, Brendan; Zeng, Zhuo; Shreeve, Jean'neM. (2009). "Azoles as Reactive Nucleophiles with Cyclic Perfluoroalkenes". Chemistry. 15 (40): 10554–62. doi:10.1002/chem.200901508. PMID 19746368.
- ^ a b c Cracowski, Jean-Marc; Sharma, Babloo; Brown, Dakarai K.; Christensen, Kenneth; Lund, Benjamin R.; Smith, Dennis W. (2012). "Perfluorocyclopentenyl (PFCP) Aryl Ether Polymers via Polycondensation of Octafluorocyclopentene with Bisphenols". Macromolecules. 45 (2): 766–71. Bibcode:2012MaMol..45..766C. doi:10.1021/ma2024599.
- ^ a b Sharma, Babloo; Hill, Sarah C.; Liff, Shawna M.; Pennington, William T.; Smith, Dennis W. (2014). "Perfluorocyclohexenyl aryl ether polymers via polycondensation of decafluorocyclohexene with bisphenols". Journal of Polymer Science Part A: Polymer Chemistry. 52 (2): 232–8. Bibcode:2014JPoSA..52..232S. doi:10.1002/pola.26995.
- Perfluorinated compounds
- Cycloalkenes



