Porphyrin

Porphyrins (/ˈpɔːrfərɪn/ POR-fər-in) are a group of heterocyclic macrocycle organic compounds, composed of four modified pyrrole subunits interconnected at their α carbon atoms via methine bridges (=CH−). The parent of porphyrin is porphine, a rare chemical compound of exclusively theoretical interest. Substituted porphines are called porphyrins.[1] With a total of 26 π-electrons, of which 18 π-electrons form a planar, continuous cycle, the porphyrin ring structure is often described as aromatic.[2][3] One result of the large conjugated system is that porphyrins typically absorb strongly in the visible region of the electromagnetic spectrum, i.e. they are deeply colored. The name "porphyrin" derives from the Greek word πορφύρα (porphyra), meaning purple.[4]
Metal complexes derived from porphyrins occur naturally. One of the best-known families of porphyrin complexes is heme, the pigment in red blood cells, a cofactor of the protein hemoglobin.
Complexes of porphyrins[]
- Representative porphyrins and derivatives
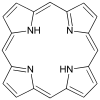
Porphin is the simplest porphyrin, a rare compound of theoretical interest.

Derivatives of protoporphyrin IX are common in nature, the precursor to hemes.

Octaethylporphyrin (H2OEP) is a synthetic analogue of protoporphyrin IX. Unlike the natural porphyrin ligands, OEP2− is highly symmetrical.
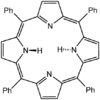
Tetraphenylporphyrin (H2TPP)is another synthetic analogue of protoporphyrin IX. Unlike the natural porphyrin ligands, TPP2− is highly symmetrical. Another difference is that its methyne centers are occupied by phenyl groups.

Simplified view of heme, a complex of a protoporphyrin IX.
Porphyrins are the conjugate acids of ligands that bind metals to form complexes. The metal ion usually has a charge of 2+ or 3+. A schematic equation for these syntheses is shown:
- H2porphyrin + [MLn]2+ → M(porphyrinate)Ln−4 + 4 L + 2 H+, where M = metal ion and L = a ligand
A porphyrin without a metal-ion in its cavity is a free base. Some iron-containing porphyrins are called hemes. Heme-containing proteins, or hemoproteins, are found extensively in nature. Hemoglobin and myoglobin are two O2-binding proteins that contain iron porphyrins. Various cytochromes are also hemoproteins.
Related species[]
A benzoporphyrin is a porphyrin with a benzene ring fused to one of the pyrrole units. e.g. verteporfin is a benzoporphyrin derivative.[5]
Several other heterocycles are related to porphyrins. These include corrins, chlorins, bacteriochlorophylls, and corphins. Chlorins (2,3-dihydroporphyrin) are more reduced, contain more hydrogen than porphyrins, i.e. one pyrrole has been converted to a pyrroline. This structure occurs in chlorophylls. Replacement of two of the four pyrrolic subunits with pyrrolinic subunits results in either a bacteriochlorin (as found in some photosynthetic bacteria) or an isobacteriochlorin, depending on the relative positions of the reduced rings. Some porphyrin derivatives follow Hückel's rule, but most do not.[citation needed]
Natural formation[]
A geoporphyrin, also known as a petroporphyrin, is a porphyrin of geologic origin.[6] They can occur in crude oil, oil shale, coal, or sedimentary rocks.[6][7] Abelsonite is possibly the only geoporphyrin mineral, as it is rare for porphyrins to occur in isolation and form crystals.[8]
Synthesis[]
Biosynthesis[]
In non-photosynthetic eukaryotes such as animals, insects, fungi, and protozoa, as well as the α-proteobacteria group of bacteria, the committed step for porphyrin biosynthesis is the formation of δ-aminolevulinic acid (δ-ALA, 5-ALA or dALA) by the reaction of the amino acid glycine with succinyl-CoA from the citric acid cycle. In plants, algae, bacteria (except for the α-proteobacteria group) and archaea, it is produced from glutamic acid via glutamyl-tRNA and glutamate-1-semialdehyde. The enzymes involved in this pathway are glutamyl-tRNA synthetase, glutamyl-tRNA reductase, and glutamate-1-semialdehyde 2,1-aminomutase. This pathway is known as the C5 or Beale pathway.
Two molecules of dALA are then combined by porphobilinogen synthase to give porphobilinogen (PBG), which contains a pyrrole ring. Four PBGs are then combined through deamination into hydroxymethyl bilane (HMB), which is hydrolysed to form the circular tetrapyrrole uroporphyrinogen III. This molecule undergoes a number of further modifications. Intermediates are used in different species to form particular substances, but, in humans, the main end-product protoporphyrin IX is combined with iron to form heme. Bile pigments are the breakdown products of heme.
The following scheme summarizes the biosynthesis of porphyrins, with references by EC number and the OMIM database. The porphyria associated with the deficiency of each enzyme is also shown:

Laboratory synthesis[]

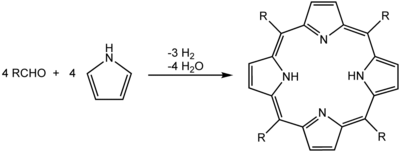
One of the most common syntheses for porphyrins is the Rothemund reaction, first reported in 1936,[9][10] which is also the basis for more recent methods described by Adler and Longo.[11] The general scheme is a condensation and oxidation process starting with pyrrole and an aldehyde.
Isomeric Porphyrins[]
The first synthetic porphyrin isomer was reported by Emanual Vogel and Coworkers in 1986. This isomer [18]porphyrin-(2.0.2.0) is named as porphycene, and the central N4 Cavity forms a rectangle shape as shown in figure.[12] Porphycenes showed interesting photophysical behavior and found versatile compound towards the photodynamic therapy.[13] This inspired Vogel and Sessler to took up the challenge of preparing [18]porphyrin-(2.1.0.1) and named it as Corrphycene or Porphycerin.[14] The third porphyrin that is [18]porphyrin-(2.1.1.0), was reported by Callot and Vogel-Sessler. Vogel and coworkers reported successful isolation of [18]Porphyrin-(3.0.1.0) or Isoporphycene.[15] The Japanese scientist Furuta[16] and Polish scientist Latos-Grażyński[17] almost simultaneously reported the N-Confused porphyrins. The inversion of one of the pyrrolic subunits in the macrocyclic ring resulted to face one of the nitrogen atom outside of the core of the macrocycle.

Applications[]
Photodynamic therapy[]
Porphyrins have been evaluated in the context of photodynamic therapy (PDT) since they strongly absorb light, which is then converted to energy and heat in the illuminated areas.[18] This technique has been applied in macular degeneration using verteporfin.[19]
PDT is considered a noninvasive cancer treatment, involving the interaction between light of a determined frequency, a photo-sensitizer, and oxygen. This interaction produces the formation of a highly reactive oxygen species (ROS), usually singlet oxygen, as well as superoxide anion, free hydroxyl radical, or hydrogen peroxide.[20] These high reactive oxygen species react with susceptible cellular organic biomolecules such as; lipids, aromatic amino acids, and nucleic acid heterocyclic bases, to produce oxidative radicals that damage the cell, possibly inducing apoptosis or even necrosis.[21]
Organic geochemistry[]
The field of organic geochemistry had its origins in the isolation of porphyrins from petroleum.[citation needed] This finding helped establish the biological origins of petroleum. Petroleum is sometimes "fingerprinted" by analysis of trace amounts of nickel and vanadyl porphyrins.[citation needed]
Toxicology[]
Heme biosynthesis is used as biomarker in environmental toxicology studies. While excess production of porphyrins indicate organochlorine exposure, lead inhibits ALA dehydratase enzyme.[22]
Potential applications[]
Biomimetic catalysis[]
Although not commercialized, metalloporphyrin complexes are widely studied as catalysts for the oxidation of organic compounds. Particularly popular for such laboratory research are complexes of meso-tetraphenylporphyrin and octaethylporphyrin. Complexes with Mn, Fe, and Co catalyze a variety of reactions of potential interest in organic synthesis. Some complexes emulate the action of various heme enzymes such as cytochrome P450, lignin peroxidase.[23][24] Metalloporphyrins are also studied as catalysts for water splitting, with the purpose of generating molecular hydrogen and oxygen for fuel cells.[25]
Molecular electronics and sensors[]
Porphyrin-based compounds are of interest as possible components of molecular electronics and photonics.[26] Synthetic porphyrin dyes have been incorporated in prototype dye-sensitized solar cells.[27][28]
Metalloporphyrins have been investigated as sensors.[29]
Phthalocyanines, which are structurally related to porphyrins, are used in commerce as dyes and catalysts, but porphyrins are not.
Supramolecular chemistry[]


Porphyrins are often used to construct structures in supramolecular chemistry. These systems take advantage of the Lewis acidity of the metal, typically zinc. An example of a host–guest complex that was constructed from a macrocycle composed of four porphyrins.[31] A guest-free base porphyrin is bound to the center by coordination with its four-pyridine substituents.
Theoretical interest in aromaticity[]
Porphyrinoid macrocycles can show variable aromaticity.[32] An Hückel aromatic porphyrin is porphycene.[33] antiaromatic, Mobius aromatic, and non aromatic porphyrinoid macrocycles are known.[34]
See also[]
- A porphyrin-related disease: porphyria
- Porphyrin coordinated to iron: heme
- A heme-containing group of enzymes: Cytochrome P450
- Porphyrin coordinated to magnesium: chlorophyll
- The one-carbon-shorter analogues: corroles, including vitamin B12, which is coordinated to a cobalt
- Corphins, the highly reduced porphyrin coordinated to nickel that binds the Cofactor F430 active site in methyl coenzyme M reductase (MCR)
- Nitrogen-substituted porphyrins: phthalocyanine
Gallery[]

Lewis structure for meso-tetraphenylporphyrin
UV–vis readout for meso-tetraphenylporphyrin

Light-activated porphyrin. Monatomic oxygen. Cellular aging
References[]
- ^ Rayati, Saeed; Malekmohammadi, Samira (2016). "Catalytic activity of multi-wall carbon nanotube supported manganese (III) porphyrin: an efficient, selective and reusable catalyst for oxidation of alkenes and alkanes with urea–hydrogen peroxide". Journal of Experimental Nanoscience. 11 (11): 872. Bibcode:2016JENan..11..872R. doi:10.1080/17458080.2016.1179802.
- ^ Ivanov, Alexander S.; Boldyrev, Alexander I. (2014). "Deciphering aromaticity in porphyrinoids via adaptive natural density partitioning". Organic & Biomolecular Chemistry. 12 (32): 6145–6150. doi:10.1039/C4OB01018C. PMID 25002069.
- ^ Lash, Timothy D. (2011). "Origin of aromatic character in porphyrinoid systems". Journal of Porphyrins and Phthalocyanines. 15 (11n12): 1093–1115. doi:10.1142/S1088424611004063.
- ^ Harper, Douglas; Buglione, Drew Carey. "porphyria (n.)". The Online Etymology Dictionary. Retrieved 14 September 2014.
- ^ Scott, L. J.; Goa, K. L. (2000). "Verteporfin". Drugs & Aging. 16 (2): 139–146, discussion 146–8. doi:10.2165/00002512-200016020-00005. PMID 10755329.
- ^ Jump up to: a b Karl M. Kadish, ed. (1999). The Porphyrin Handbook. Elsevier. p. 381. ISBN 9780123932006.
- ^ Zhang, Bo; Lash, Timothy D. (September 2003). "Total synthesis of the porphyrin mineral abelsonite and related petroporphyrins with five-membered exocyclic rings". Tetrahedron Letters. 44 (39): 7253. doi:10.1016/j.tetlet.2003.08.007.
- ^ Mason, G. M.; Trudell, L. G.; Branthaver, J. F. (1989). "Review of the stratigraphic distribution and diagenetic history of abelsonite". Organic Geochemistry. 14 (6): 585. doi:10.1016/0146-6380(89)90038-7.
- ^ P. Rothemund (1936). "A New Porphyrin Synthesis. The Synthesis of Porphin". J. Am. Chem. Soc. 58 (4): 625–627. doi:10.1021/ja01295a027.
- ^ P. Rothemund (1935). "Formation of Porphyrins from Pyrrole and Aldehydes". J. Am. Chem. Soc. 57 (10): 2010–2011. doi:10.1021/ja01313a510.
- ^ A. D. Adler; F. R. Longo; J. D. Finarelli; J. Goldmacher; J. Assour; L. Korsakoff (1967). "A simplified synthesis for meso-tetraphenylporphine". J. Org. Chem. 32 (2): 476. doi:10.1021/jo01288a053.
- ^ Vogel, E; K6cher, M. (March 1986). "Porphycene—a Novel Porphin Isomer". Angewandte Chemie. 25 (3): 257. doi:10.1002/anie.198602571.
- ^ THOMAS J., DOUGHERTY (2001). "Basic principles of photodynamic therapy". J. Porphyrins Phthalocyanines. 5 (2): 105. doi:10.1002/jpp.328.
- ^ Prof. Dr. Emanuel, Vogel; Prof. Dr. Roger, Guilard (November 1993). "New Porphycene Ligands: Octaethyl‐ and Etioporphycene (OEPc and EtioPc)—Tetra‐ and Pentacoordinated Zinc Complexes of OEPc". Angewandte Chemie International Edition. 32 (11): 1600. doi:10.1002/anie.199316001.
- ^ Vogel, Emanuel; Scholz, Peter; Demuth, Ralf; Erben, Christoph; Bröring, Martin; Schmickler, Hans; Lex, Johann; Hohlneicher, Georg; Bremm, Dominik; Wu, Yun-Dong (4 October 1999). "Isoporphycene: The Fourth Constitutional Isomer of Porphyrin with an N4 Core—Occurrence of E/Z Isomerism". Angewandte Chemie International Edition. 38 (19): 2919–2923. doi:10.1002/(SICI)1521-3773(19991004)38:19<2919::AID-ANIE2919>3.0.CO;2-W. PMID 10540393.
- ^ Hiroyuki, Furuta (1994). ""N-Confused Porphyrin": A New Isomer of Tetraphenylporphyrin". J. Am. Chem. Soc. 116 (2): 767. doi:10.1021/ja00081a047.
- ^ Dr. Lechoslaw, Latos‐Grażyński (18 April 1994). "Tetra‐p‐tolylporphyrin with an Inverted Pyrrole Ring: A Novel Isomer of Porphyrin". Angewandte Chemie International Edition. 33 (7): 779. doi:10.1002/anie.199407791.
- ^ Giuntini, Francesca; Boyle, Ross; Sibrian-Vazquez, Martha; Vicente, M. Graca H. (2014). "Porphyrin conjugates for cancer therapy". In Kadish, Karl M.; Smith, Kevin M.; Guilard, Roger (eds.). Handbook of Porphyrin Science. 27. pp. 303–416.
- ^ Wormald R, Evans J, Smeeth L, Henshaw K (2007). "Photodynamic therapy for neovascular age-related macular degeneration" (PDF). Cochrane Database Syst Rev (3): CD002030. doi:10.1002/14651858.CD002030.pub3. PMID 17636693.
- ^ Price, M., Terlecky, S. R. and Kessel, D. (2009), A Role for Hydrogen Peroxide in the Pro‐apoptotic Effects of Photodynamic Therapy. Photochemistry and Photobiology, 85: 1491-1496. doi:10.1111/j.1751-1097.2009.00589.x
- ^ Singh, S., Aggarwal, A., N. V. S. Dinesh K. Bhupathiraju, Arianna, G., Tiwari, K., & Drain, C. M. (2015). Glycosylated Porphyrins, Phthalocyanines, and Other Porphyrinoids for Diagnostics and Therapeutics. Chemical Reviews, 115(18), 10261-10306. doi:10.1021/acs.chemrev.5b00244
- ^ Walker, C. H.; Silby, R. M.; Hopkin, S. P.; Peakall; D.B. (2012). Principles of Ecotoxicology. Boca Raton, FL: CRC Press. p. 182. ISBN 978-1-4665-0260-4.
- ^ Huang, Xiongyi; Groves, John T. (2018). "Oxygen Activation and Radical Transformations in Heme Proteins and Metalloporphyrins". Chemical Reviews. 118 (5): 2491–2553. doi:10.1021/acs.chemrev.7b00373. PMC 5855008. PMID 29286645.
- ^ Karl M. Kadish; Kevin M. Smith; Roger Guilard, eds. (2012). Handbook of porphyrin science with applications to chemistry, physics, materials science, engineering, biology and medicine. Singapore: World Scientific. ISBN 9789814335492.
- ^ Zhang, Wei; Lai, Wenzhen; Cao, Rui (22 February 2017). "Energy-Related Small Molecule Activation Reactions: Oxygen Reduction and Hydrogen and Oxygen Evolution Reactions Catalyzed by Porphyrin- and Corrole-Based Systems". Chemical Reviews. 117 (4): 3717–3797. doi:10.1021/acs.chemrev.6b00299. ISSN 0009-2665. PMID 28222601.
- ^ By Lewtak, Jan P.; Gryko, Daniel T. (2012). "Synthesis of π-extended porphyrins via intramolecular oxidative coupling". Chemical Communications. 48 (81): 10069–10086. doi:10.1039/c2cc31279d. PMID 22649792.
- ^ Michael G. Walter; Alexander B. Rudine; Carl C. Wamser (2010). "Porphyrins and phthalocyanines in solar photovoltaic cells". Journal of Porphyrins and Phthalocyanines. 14 (9): 759–792. doi:10.1142/S1088424610002689.
- ^ Aswani Yella; Hsuan-Wei Lee; Hoi Nok Tsao; Chenyi Yi; Aravind Kumar Chandiran; Md.Khaja Nazeeruddin; Eric Wei-Guang Diau; Chen-Yu Yeh; Shaik M Zakeeruddin; Michael Grätzel (2011). "Porphyrin-Sensitized Solar Cells with Cobalt (II/III)–Based Redox Electrolyte Exceed 12 Percent Efficiency". Science. 334 (6056): 629–634. Bibcode:2011Sci...334..629Y. doi:10.1126/science.1209688. PMID 22053043. S2CID 28058582.
- ^ Ding, Yubin; Zhu, Wei-Hong; Xie, Yongshu (2017). "Development of Ion Chemosensors Based on Porphyrin Analogues". Chemical Reviews. 117 (4): 2203–2256. doi:10.1021/acs.chemrev.6b00021. PMID 27078087.
- ^ Pham, Tuan Anh; Song, Fei; Alberti, Mariza N.; Nguyen, Manh-Thuong; Trapp, Nils; Thilgen, Carlo; Diederich, François; Stöhr, Meike (2015). "Heat-induced formation of one-dimensional coordination polymers on Au(111): An STM study" (PDF). Chem. Commun. 51 (77): 14473–6. doi:10.1039/C5CC04940G. PMID 26278062.
- ^ Jump up to: a b Sally Anderson; Harry L. Anderson; Alan Bashall; Mary McPartlin; Jeremy K. M. Sanders (1995). "Assembly and Crystal Structure of a Photoactive Array of Five Porphyrins". Angew. Chem. Int. Ed. Engl. 34 (10): 1096–1099. doi:10.1002/anie.199510961.
- ^ Schleyer, Paul v. R.; Wu, Judy I.; Fernández, Israel (3 December 2012). "Description of Aromaticity in Porphyrinoids". J. Am. Chem. Soc. 135 (1): 315–21. doi:10.1021/ja309434t. PMID 23205604.
- ^ Kadish, Karl M.; Smith, Kevin M.; Guilard, Roger. The Porphyrin Handbook. Academic Press. ISBN 0123932009.
- ^ Yoon, Zin Seok; Osuka, Atsuhiro; Kim, Dongho (2009). "Möbius aromaticity and antiaromaticity in expanded porphyrins". Nature Chemistry. 1 (2): 113–22. Bibcode:2009NatCh...1..113Y. doi:10.1038/nchem.172. PMID 21378823.
External links[]
| Wikimedia Commons has media related to Porphyrins. |
- Biomolecules
- Metabolism
- Photosynthetic pigments
- Chelating agents
- Porphyrins