Rongalite
| |
| Names | |
|---|---|
| IUPAC name
Sodium hydroxymethanesulfinate
| |
| Other names
Sodium formaldehydesulfoxylate, sodium oxymethylene sulfoxylate, Brüggolit
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.219 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
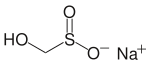
| CH3NaO3S | |
| Molar mass | 118.10 g/mol 154.14 g/mol, dihydrate |
| Appearance | colorless crystals |
| Density | 1.75 g/cm3, dihydrate |
| Melting point | 64.5 °C (148.1 °F; 337.6 K) dihydrate |
| 600 g/L, dihydrate (approximate) | |
| Acidity (pKa) | decomposes at low pH |
| Structure | |
| pyramidal at S | |
| Hazards | |
| GHS labelling: | |

| |
Signal word
|
Warning |
| H341 | |
| P201, P202, P281, P308+P313, P405, P501 | |
| Related compounds | |
Related compounds
|
SO32−, CH2O |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Rongalite is a chemical compound with the molecular formula Na+HOCH2SO2−. This salt has many additional names, including Rongalit, sodium hydroxymethylsulfinate, sodium formaldehyde sulfoxylate, and Bruggolite. It is listed in the European Cosmetics Directive as sodium oxymethylene sulfoxylate (INCI). It is water-soluble and generally sold as the dihydrate. The compound and its derivatives are widely used in the dye industry.[1]
Synthesis and reactions[]
Although available commercially, the salt can be prepared from sodium dithionite and formaldehyde:
- Na2S2O4 + 2 CH2O + H2O → HO-CH2-SO3Na + HO-CH2-SO2Na
This reaction proceeds quantitatively, such that dithionite can be determined by its conversion to Rongalite, which is far less O2-sensitive and thus easier to handle.
The hydroxymethanesulfinate ion is unstable in solution towards decomposition to formaldehyde and sulfite. Addition of at least one equivalent of formaldehyde pushes the equilibrium towards the side of the adduct and reacts further to give the bis-(hydroxymethyl)sulfone. Such solutions are shelf-stable indefinitely.
Sodium hydroxymethanesulfinate was originally developed in the early 20th century for the textile industry as a shelf-stable source of sulfoxylate ion, where the latter can be generated at will. In use, when sodium hydroxymethanesulfinate is made acidic, the reducing sulfoxylate ion and formaldehyde are released in equimolar amounts. For safety reasons the generation of formaldehyde must be taken into consideration when used industrially.
NaHOCH2SO2 can essentially be considered to be a source of SO22−. As such it is used both as a reducing agent and as a reagent to introduce SO2 groups into organic molecules. Treatment of elemental Se and Te with NaHOCH2SO2 gives solutions containing the corresponding Na2Sex and Na2Tex, where x is approximately 2. As a nucleophile, NaHOCH2SO2 reacts with alkylating agents to give sulfones.
- HO-CH2-SO2Na + 2 C6H5CH2Br → [C6H5CH2]2SO2 + NaBr + CH2O + HBr
Occasionally, alkylation will occur also at oxygen, thus α,α'-dibromoxylene gives both the sulfone and the isomeric sulfinate ester.
Use[]
The original use of the compound was as industrial bleaching agent and as a reducing agent for vat dyeing.[1] Another large-scale use is as a reducing agent in redox-initiator systems for emulsion polymerization. One of the typical redox pair examples is t-butyl peroxide. A niche use is its use as water conditioner for aquaria as it rapidly reduces chlorine and chloramine and reacts with ammonia to form the innocuous aminomethylsulfinate ion.[2] It is also used as an antioxidant in pharmaceutical formulation.
The compound has been used increasingly in commercial cosmetic hair dye colour removers despite the generation of formaldehyde, a known human carcinogen.
It has a variety of specialized applications in organic synthesis.[3][4]
Related compounds[]
The zinc complex Zn(HOCH2SO2)2 is marketed under the trademarks Decroline, Decolin, and Safolin. This compound is an additive in polymers and textiles.[5]
Sodium hydroxymethanesulfinate is called Rongalite C. Calcium hydroxymethanesulfinate is called Rongalite H.
References[]
- ^ a b D. Schubart "Sulfinic Acids and Derivatives" in Ullmann's Encyclopedia of Industrial Chemistry, 2012, Wiley-VCH, Weinheim. doi:10.1002/14356007.a25_461
- ^ EP patent 0278515, Günter Ritter, "Chloramine removing means", issued 1988-08-17, assigned to Tetra-Werke
- ^ Dittmer, Donald C "Sodium Hydroxymethanesulfinate" e-EROS Encyclopedia of Reagents for Organic Synthesis, 2001. doi:10.1002/047084289X.rs083
- ^ Kotha, Sambasivarao; Khedkar, Priti (2011). "Rongalite: A Useful Green Reagent in Organic Synthesis". Chemical Reviews. 112 (3): 1650–1680. doi:10.1021/cr100175t. PMID 22107104.
- ^ Masciocchi, N.; Rigamonti, C.; Maspero, A. (2005). "Poly[di-μ3-hydroxymethanesulfinato-zinc(II)]". Acta Crystallographica E. 61 (12): m2683–m2685. doi:10.1107/S1600536805038237.
Further reading[]
- Holleman, A. F.; Wiberg, E. (2001). Inorganic Chemistry. San Diego: Academic Press. ISBN 978-0-12-352651-9.
- Tschugaeff, L.; Chlopin, W. (1914). "Beiträge zur Kenntnis des Reduktionsvermögens der schwefligen Säure. I. Einwirkung von Natriumhydrosulfit auf Tellur und Selen". Chemische Berichte. 47 (1): 1269–1275. doi:10.1002/cber.191404701202.
- Steudel, R.; Münchow, V. (1992). "Sulphur compounds: CLIX. Determination of Dithionite (S2O42−) and Hydroxymethanesulphinate (HOCH2SO2−; Rongalite) by Ion-Pair Chromatography". Journal of Chromatography A. 623 (1): 174–177. doi:10.1016/0021-9673(92)85314-J.
- Organic sodium salts
- Sulfinates
- Reducing agents