Roskamp reaction
This article has multiple issues. Please help or discuss these issues on the talk page. (Learn how and when to remove these template messages)
|
The Roskamp reaction was first discovered by and co-workers in 1989.[1] This reaction is very useful in synthesizing from aldehydes and , using various Lewis acids as catalysts (such as BF3, SnCl2, GeCl2).
The two most noteworthy aspects of Roskamp reaction are mild conditions and selectivity. Usually it only takes no more than one hour at room temperature to completely convert the starting materials to products.
Background[]
The first paper about Roskamp Reaction was published in Journal of Organic Chemistry in 1989. At the very beginning, they proposed that the reaction would convert aldehydes to alkenes via a pseudo-Wittig type Reaction. However, β-keto esters were the only products they observed.
In their first published paper, they observed that aliphatic aldehydes always have higher yield than aromatic aldehydes because of enolization. Also, the mild reaction conditions shows advantages in preventing side reactions and increasing functional group tolerance.


Then in 1992, Roskamp and co-workers expanded the scope of diazoacetate to , and .[2]

Mechanism[]
Diazo compounds are ambiphilic reagents. According to its resonance structure, the carbon adjacent to the diazo group has partial negative charge.[3] If R’ = H, this can be regarded as a hydride-transfer process.

Roskamp-Feng Reaction[]
Because the α-diazocarbonyl compounds can serve as a Michael-type or aldo-type nucleophile, it is a good way to develop asymmetric reactions. In 2009, Keiji Maruoka[4] from Kyoto University reported Lewis acid catalyzed asymmetric Roskamp Reaction.[5] The chiral information is introduced by chiral auxiliaries from substrates.

Despite being catalytic, the previous examples rely on chiral auxiliaries for chiral information introduction. The Feng group developed chiral Sc-catalyzed enantioselective Roskamp Reaction which is a much more economical method.
The Roskamp-Feng reaction, named after Eric J. Roskamp and Xiaoming Feng, is an enantioselective chemical reaction between aldehydes and alkyl diazoacetates to prepare chiral β-keto esters.
Roskamp Reaction is well known for its mild reaction condition, high conversion rates as well as broad substrate scope. In 2010, Xiaoming Feng used a special N,N’-dioxide-Sc(OTF)3 chiral ligands (0.05 mol %) to realize the first case of asymmetric Roskamp reaction. The reaction was performed well over a series of substrates, giving the desired products chemoselectively in excellent yields (up to 99%) and enantioselectivities (up to 98% ee) under mild conditions.[6]
Their best ligand:
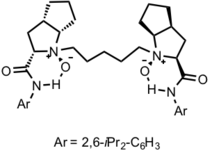
The disadvantage is that the substrate can only be aromatic aldehydes. However, Roskamp-Feng Reaction is still very meaningful in synthesizing chiral 1,3-diol by reduction of keto ester, which is a very useful building block in natural product synthesis.

Chiral N,N’-dioxide ligands
Structure:

Advantages:
- Ready availability
- The reactions of tertiary amines or pyridine with peracids such as m-CPBA and hydrogen peroxide easily generate the corresponding N-oxides.
- Compatible with a wide variety of metals, including (Sc3+, Y3+, La3+, Nd3+, Sm3+, Eu3+, Gd3+, Yb3+), transition metals (Ti4+, Fe3+, Fe2+, Co2+, Ni2+, Cu2+, Ag+) and main group metals(Mg2+).
- In a number of asymmetric reactions, N,N′- dioxide–metal complexes allow for a remarkable stereochemical outcome under mild reaction conditions.
Other asymmetric Roskamp reactions[]
In 2012, [7] from Sungkyunkwan University developed a catalytic, asymmetric Roskamp Reaction with broad applicability.[8] They used the oxazaborolidinium ion Lewis acid catalysts, which are generated from the corresponding oxazaborolidine by protonation with triflic acid.

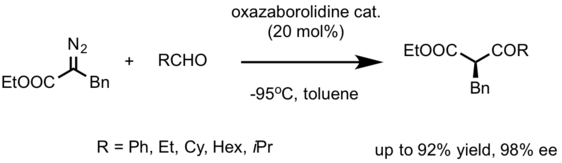
The advantage of this reaction compared to Feng's, is its broad scope of aldehydes. Long-chain heptaldehyde, as well as more sterically hindered isopropyl and all successfully reacted with diazoester to provide the corresponding products.
In 2015, the same group reported asymmetric Roskamp reaction of the α-aryl diazo Weinreb amide, using the same chiral catalyst.[9] The reaction is useful because Weinreb amide can be converted to ketones easily.

See also[]
- Meerwein arylation - also sees substitution of a diazonium compound by a carbon centre
References[]
- ^ Holmquist, Christopher R.; Roskamp, Eric J. (1989-07-01). "A selective method for the direct conversion of aldehydes into .beta.-keto esters with ethyl diazoacetate catalyzed by tin(II) chloride". The Journal of Organic Chemistry. 54 (14): 3258–3260. doi:10.1021/jo00275a006. ISSN 0022-3263.
- ^ Holmquist, Christopher R. (1992-02-25). "Tin(II) chloride catalyzed addition of diazo sulfones, diazo phosphine oxides, and diazo phosphonates to aldehydes". Tetrahedron Letters. 33 (9): 1131–1134. doi:10.1016/S0040-4039(00)91877-X.
- ^ Zhang, Yan; Wang, Jianbo (2009-09-02). "Recent development of reactions with α-diazocarbonyl compounds as nucleophiles". Chemical Communications (36): 5350–5361. doi:10.1039/B908378B. ISSN 1364-548X. PMID 19724784.
- ^ "Professor Maruoka - Maruoka Group, Department of Chemistry, Graduate School of Science, Kyoto University".
- ^ Hashimoto, Takuya; Miyamoto, Hisashi; Naganawa, Yuki; Maruoka, Keiji (2009-08-19). "Stereoselective Synthesis of α-Alkyl-β-keto Imides via Asymmetric Redox C−C Bond Formation between α-Alkyl-α-diazocarbonyl Compounds and Aldehydes". Journal of the American Chemical Society. 131 (32): 11280–11281. doi:10.1021/ja903500w. ISSN 0002-7863. PMID 19630397.
- ^ Li, Wei; Wang, Jun; Hu, Xiaolei; Shen, Ke; Wang, Wentao; Chu, Yangyang; Lin, Lili; Liu, Xiaohua; Feng, Xiaoming (2010-06-30). "Catalytic Asymmetric Roskamp Reaction of α-Alkyl-α-diazoesters with Aromatic Aldehydes: Highly Enantioselective Synthesis of α-Alkyl-β-keto Esters". Journal of the American Chemical Society. 132 (25): 8532–8533. doi:10.1021/ja102832f. ISSN 0002-7863. PMID 20527752.
- ^ "Natural product synthesis laboratory".
- ^ Gao, Lizhu; Kang, Byung Chul; Hwang, Geum-Sook; Ryu, Do Hyun (2012-08-13). "Enantioselective Synthesis of α-Alkyl-β-ketoesters: Asymmetric Roskamp Reaction Catalyzed by an Oxazaborolidinium Ion". Angewandte Chemie International Edition. 51 (33): 8322–8325. doi:10.1002/anie.201204350. ISSN 1521-3773. PMID 22821683.
- ^ Shin, Sung Ho; Baek, Eun Hee; Hwang, Geum-Sook; Ryu, Do Hyun (2015-10-02). "Enantioselective Synthesis of syn-α-Aryl-β-hydroxy Weinreb Amides: Catalytic Asymmetric Roskamp Reaction of α-Aryl Diazo Weinreb Amides". Organic Letters. 17 (19): 4746–4749. doi:10.1021/acs.orglett.5b02268. ISSN 1523-7060. PMID 26393875.
- Chemical reactions
- Name reactions